Introduction
Cardiovascular complications are considered as the main cause of mortality in patients with End-Stage Renal Disease (ESRD). Cardiovascular Disease (CVD) includes disorders of the Left Ventricular Hypertrophy (LVH) of the heart which is the most frequent cardiac alteration in ESRD. Galectin-3 (GAL-3), a β-galactoside-binding protein has been proposed to be a new clinical biomarker that reflects cardiac fibrosis in patients with Heart Failure (HF).
Aim
To evaluate the relationship between GAL-3 and biochemical parameters in Peritoneal Dialysis (PD) patients with and without LVH.
Materials and Methods
This cross-sectional study enrolled 45 patients (25 women and 20 men) with ESRD who were categorised as having CVD with (n=12) or without (n=33) LVH. Demographic, biochemical and clinical characteristics of 45 patients were analysed. The relationship of plasma GAL-3 levels was analysed with the biochemical parameters for both the groups of patients. For comparison between groups, Student unpaired t-test was used for the data of normal distribution while Mann-Whitney test was used for data of non-Gaussian distribution. Pearson’s correlation test was performed to examine various correlations.
Results
Significantly high number (83.3%) of female patients were observed in ESRD with LVH. The groups did not differ significantly in their demographic, and biochemical and clinical parameters. There was significant increase in Left Ventricular End-Diastolic Diameters (LVEDD), Left Ventricular (LV) mass and LV mass index in patients with LVH as compared to the patients without LVH. The levels of GAL-3 showed slight increase (91±23.98 ng/mL) levels in LVH patients as compared to the patients without LVH (83.68±32.8 ng/mL). Exponential positive correlation between serum levels of GAL-3 and creatinine in ESRD patients without LVH (r=0.563, p=0.001). GAL-3 also showed positive correlations with urea without (r=0.563, p=0.001) as well as and uric acid (r=0.416, p=0.0178) for ESRD patients without LVH. However, GAL-3 showed no association with uric acid and urea (r=0.04487, p=0.896; r=0.2383, p=0.48) in ESRD patients with LVH.
Conclusion
GAL-3 positively correlated to the biochemical parameters in ESRD patients. Patients with LVH only showed positive correlation between GAL-3 and creatinine. Moreover, GAL-3 could not be used as the biomarker because it did not correlate with established diagnostic parameter like LV mass and LV mass index. Hence, in this study GAL-3 is not a potential clinical biomarker for the progression of cardiovascular complications in ESRD patients. Overall, these data reflect the need for further investigation of GAL-3 to HF in patients with ESRD.
Introduction
Galectin-3 is a 31 KDa β-galctoside-binding protein produced by macrophages and other cells. It has been shown to play a role in the regulation of inflammation and fibrosis [1]. In addition, Galectin-3 has been recently reported as a biomarker for HF [2].
ESRD affects individuals throughout the world and is now recognised as a major public health problem [3,4]. The crude prevalence of ESRD in Saudi Arabia is 4% of the general population and it is estimated that the incidence of ESRD has also increased [5]. The diagnosis of ESRD requires a measured decrease in kidney function, with a Glomerular Filtration Rate (GFR) of less than 60 mL/min per 1.73 m2 and/or kidney damage for three months or more. Kidney damage refers to pathologic abnormalities evidenced by biopsy or imaging as well as alterations in urinary sediment or proteinuria (proteinuria/creatinuria >200 mg/g, albuminuria/creatinuria >30 mg/g) [6]. ESRD is the final common pathway for all renal diseases; may occur due to ischemia, hypoxia, proteinuria, hypertension and in presence of predisposing factors (smoking, diabetes, hyperlipidemia) [7-9].
ESRD has many adverse outcomes which need to be monitored and managed. A wide variety of diseases including CVD and obesity are associated with ESRD and haemodialysis [10]. Recently, a medical term, ‘Cardiorenal Syndrome’ (CRS), was coined to describe acute to chronic clinical overlapping of kidney and heart dysfunctions [11]. Dialysis and Renal Replacement Therapy (RRT) is required in end-stage disease. Dialysis is a common alternative due to limited availability of organ donors [12,13].
Dialysis may involve haemodialysis or PD. It is well established that patients with ESRD have a high incidence of CVD compared to the general population. Moreover, CVD is considered the major cause of morbidity in ESRD patients [14]. This association has not been fully understood and cannot be adequately explained by changes in the traditional risk factors [15]. The ESRD is also known as irreversible advanced Chronic Kidney Disease (CKD) where there is permanent loss of kidney function causing extreme mortality rates [6,16]. Continuous Ambulatory Peritoneal Dialysis (CAPD) helps in treating ascites and is a suitable alternative to dialysis [17].
LVH is considered one of major independent predictor of CVD and one of typical feature of ESRD. The most common cause of LVH is hypertrophy which results from an increased preload due to hypervolemia and an increased afterload due to peripheral resistance. There is a high prevalence of CVD (40%) and ventricular hyperthrophy (70%) in patients on RRT [10,18]. Moreover, dialysis patients have three-fold increase risk of HF and high rate of mortality due to LVH [19]. A faster diagnosis is important to prevent CVD complications. Also, the etiology must be understood so that appropriate therapy is started [20,21]. As GAL-3 has been reported a clinical biomarker in HF, it would be interesting to evaluate the role of GAL-3 in ESRD patients with and without LVH. The aim of this study was to understand the role of GAL-3 in ESRD patients undergoing PD in terms of renal function testing.
Materials and Methods
Study Population
This cross-sectional study enrolled 45 patients (25 women and 20 men) with ESRD attending the nephrology clinic at King Fahad Specialist Hospital, Qassim, Saudi Arabia from January to December 2016. The research protocol was approved by Regional Research Ethics Committee- Qassim (number: 1252/33/45). Written informed consent was obtained from all participants before enrollment in the study.
Inclusion criteria: Among 72 patients, only 45 patients fulfilled the inclusion criteria, which were lack of permanent heart rhythm disorders, no diastolic-systolic dysfunction, no moderate to severe valvular disease (diagnosed with echocardiography) and absence of active inflammatory or infectious disease based on clinical evaluation and laboratory testing.
Exclusion criteria: Patients were excluded from the study if they had CVDs such as myocardial infarction, angina pectoris, valvular heart disease, hypertrophic dilated cardiomyopathy.
All patients on Continuous Ambulatory Peritoneal Dialysis (CAPD) received four exchanges per day using standard dialysis bags (8 L/day). Information on patient’s demographics and clinical parameters was obtained from medical records and also from patients themselves using a case report form.
Galectin-3 Measurements
Blood samples were collected after an overnight fast and immediately centrifuged at 3000 rpm for 15 minutes at 4°C. The samples were then stored at -70°C until being assayed. Galectin-3 levels were measured by immunoassay using ARCHITECT i2000SR (Abbott Diagnostics, Abbott Park, IL, USA) according to the manufacturer instructions. All samples were assayed in duplicate and the values were represented as mean±SD.
Patients Demographics, Risk Factors and Clinical Analysis
Patient demographics were recorded upon enrolment to the hospital. Risk factors such as BMI, Systolic and Diastolic Blood Pressures (SBP and DBP), Total Cholesterol (TC), Triglycerides (TGs), High Density Lipoprotein Cholesterol (HDL-C), Low Density Lipoprotein Cholesterol (LDL-C) and the ratio of TGs/HDL, hypertension, urea, uric acid and creatinine were estimated. Echocardiographic measurements were performed in accordance with the American Society of Echocardiography (ASE) guidelines [22].
Statistical Analysis
The statistical significance of the difference between the means of two groups of samples was assessed by the data which was expressed as mean±SD. For comparison between the groups, Student unpaired t-test was used for the data of normal distribution while Mann-Whitney test was used for data of non-Gaussian distribution. Pearson’s correlation test was performed to examine various correlations. Differences were considered to be significant when the p-value was less than 0.05 (p<0.05).
Results
Demographic, risk factors and clinical characteristics of the 45 patients are summarised in [Table/Fig-1]. Patients were classified into two groups accordingly; with LVH (n=12) and without LVH (n=33). The mean age of the patients with LVH was 41±18 years and the patients without LVH was 37±17. In patients with LVH, 83.3% were females, however, in patients without LVH 45.45% were females. The groups did not differ significantly in their BMI, SBP, DBP, TC, TGs, HDL-C, LDL-C and in the ratio of TGs/HDL [Table/Fig-1].
Demographic and clinical characteristics of patients.
| Variables | Patients with LVH (n:12) | Patients without LVH (n:33) | p-value |
|---|
| Age (years) | 41±18 | 37±17 | 0.324 |
| Gender | 10 F/2 M | 15 F/18 M | |
| BMI | 25±5.8 | 24±5.6 | 0.650 |
| SBP (mmHg) | 148.7±16.8 | 137±37.52 | 0.126 |
| DBP (mmHg) | 81.7±8.82 | 82.6±37.53 | 0.899 |
| Galectin-3 (ng/mL) | 91±23.98 | 83.68±32.80 | 0.632 |
| TC (mmol/L) | 4.03±2.80 | 3.75±1.85 | 0.756 |
| TGs (mmol/L) | 0.87±0.51 | 1.32±1.3 | 0.084 |
| HDL-C (mmol/L) | 0.99±0.87 | 0.85±0.36 | 0.612 |
| LDL-C (mmol/L) | 2.71±1.94 | 2.35±1.4 | 0.579 |
| TGs/HDL ratio | 1.13±0.57 | 1.95±2.2 | 0.062 |
| Non HDL-C (mmol/L) | 3.05±2.20 | 2.9±1.72 | 0.844 |
| Uric Acid (μmol/L) | 343±55.38 | 377.3±94.4 | 0.156 |
| Urea (mmol/L) | 17.17±6.88 | 19.54±6.42 | 0.40 |
| Creatinine (μmol/L) | 977.3±423.8 | 1013±342.1 | 0.805 |
| LVEDD (mm) | 50±6.2 | 41±5.25 | 0.001 |
| PWT (mm) | 10.55±1.81 | 12.05±12.34 | 0.598 |
| EF (%) | 51.6±15.56 | 58.97±2.7 | 0.180 |
| LV mass (g) | 198.5±35.56 | 123.7±41.47 | <0.0001 |
| LV mass index (g/m2) | 125.5±20.84 | 76.06±16.2 | <0.0001 |
BMI: Body mass index; EF: Ejection fraction; LV: Left ventricular; LVEDD: Left ventricular end diastolic diameter; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; PWT: Posterior wall thickness; TC: Total cholesterol; TGs: Triglyceride; HDL-C: High density lipoprotein cholesterol; LDL-C: Low density lipoprotein cholesterol; Non-HDL-C: Non-high density lipoprotein cholesterol and TGs/HDL-C: Triglyceride/high density lipoprotein cholesterol ratio
There was also no significant difference in both the groups for uric acid, urea and creatinine in the blood levels [Table/Fig-1]. However, there was moderate increase in LVEDD, LV mass and LV mass index in patients with LVH as compared to the patients without LVH. The patients with LVH showed slight increase (91±23.98 ng/mL) in the level of GAL-3 as compared to the patients without LVH (83.68±32.8 ng/mL) however, this increase was not significant [Table/Fig-1].
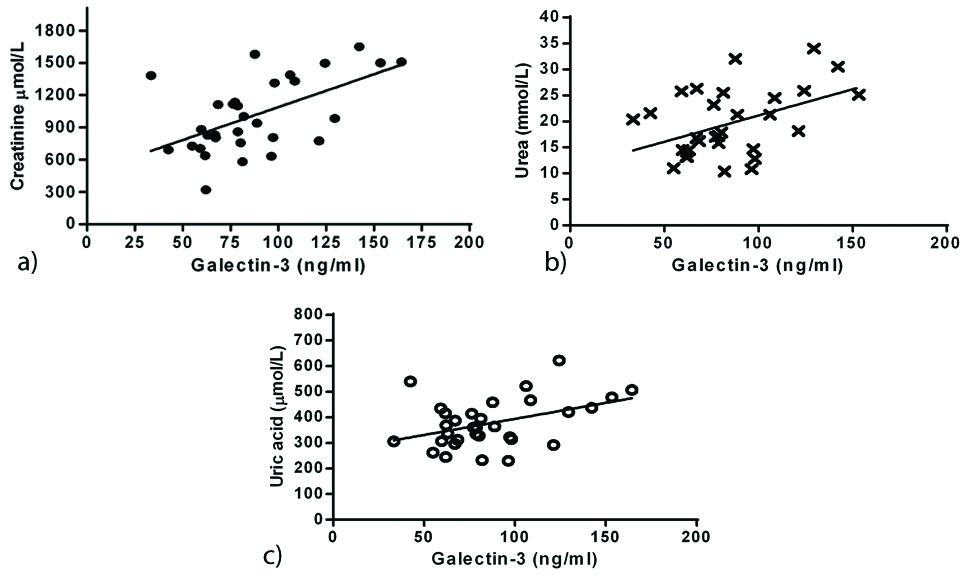
Correlations were analysed in all ESRD patients between GAL-3 and risk factors mentioned in this study. In all ESRD patients without LVH, GAL-3 showed an exponential positive correlation with creatinine ([Table/Fig-2a]; r=0.563, p=0.001), urea ([Table/Fig-2b]; r=0.446, p=0.0119) and uric acid ([Table/Fig-2c]; r=0.439, p=0.0246).
Correlation of Galectin-3 versus creatinine (a) (r=0.563, p=0.001), urea (b) (r=0.446, p=0.0119) and uric acid (c) (r=0.416, p=0.0178) in all ESRD patients without LVH.
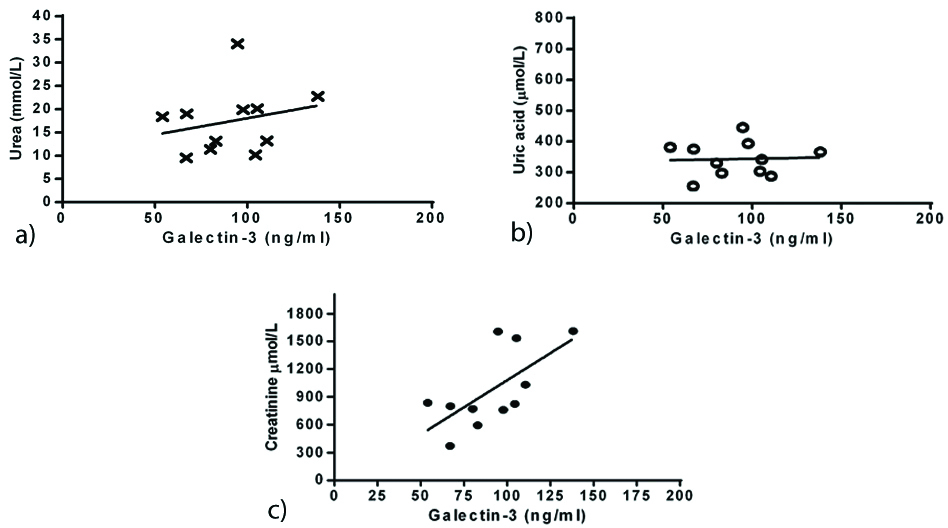
In contrast, serum levels of GAL-3 with LVH patients showed no correlation with urea ([Table/Fig-3a]; r=0.2383, p=0.4804) and uric acid ([Table/Fig-3b]; r=0.04487, p=0.896). Further analysis about this correlation revealed that only LVH group had GAL-3 association with creatinine ([Table/Fig-3c]; r=0.6624, p=0.026).
Correlation of Galectin-3 versus urea (a) (r=0.239, p=0.480), uric acid (b) (r=0.045, p=0.896) and creatinine (c) (r=0.439, p=0.0246) in all ESRD patients with LVH.
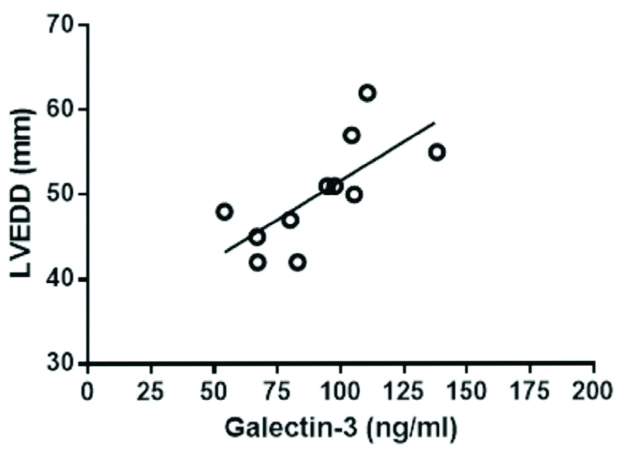
There was no significant difference in serum levels of GAL-3 among ESRD on PD either with LVH or without LVH. However, in LVH patients, higher galaectin-3 levels were associated with higher LVEDD [Table/Fig-4].
Correlation of Galectin-3 versus LVEDD (r=0.709, p=0.0145) in ESRD patients with LVH.
Discussion
Gal-3 is a novel biomarker produced by activated macrophages, and is associated with myocardial fibrosis and progression of HF [16]. It is already known that patients with ESRD have a high incidence of CVD compared to the general population [14]. LVH is considered one of major independent predictor of CVD and one of typical feature of ESRD. Hence, to understand the association between Gal-3 and ESRD patients with and without LVH, the level of serum Gal-3 was screened in these patients. In this study, demographic, risk factors and clinical characteristics of ESRD patients were also analysed. Patients with LVH included a higher number of females as compared to males. It is well established that women have lower incidence of CVD as compared to men, more specifically coronary artery diseases and acute coronary syndrome [23,24]. However, HF, stroke and atrial fibrillation are more often seen in elderly women [25-27], with an increase in prevalence of hypertension [28,29]. LV and LVH are more prevalent in hypertensive women with subclinical cardiac damage [30].
However, in patients without LVH, no remarkable difference in the ratio of male and female patients was observed (p-value not significant (0.282)). Both the groups did not differ significantly in their BMI, SBP, DBP, TC, TGs, HDL-C, LDL-C and in the ratio of TGs/HDL. Although slight higher values of Uric acid, urea and creatinine were observed in ESRD patients without LVH, however, the differences were moderately increased in LVEDD, LV mass and LV mass index in patients with LVH as compared to the patients without LVH. GAL-3 also contributes in the progression of cardiovascular remodeling along with many inflammatory and autoimmune diseases [31-34]. Increased levels of GAL-3 were detected in most of CVD patients and its prognostic values for various clinical outcome were investigated extensively [35]. It has been reported that high expression of GAL-3 levels was related with mortality in acute and chronic HF [36,37]. Increased levels of GAL-3 were estimated using immunoassay in LVH patients as compared to the patients without LVH. However, this increase was not significant. Moreover, patients with and without LVH exhibited positive correlation between GAL-3 and creatinine.
In contrast, GAL-3 did not show any association with urea and uric acid in ESRD patients with LVH. Furthermore, in ESRD patients without LVH, GAL-3 showed positive correlation with creatinine, urea and uric acid. This is further ascertained by a previously published systematic review which indicated that galectin-3 is ineffective in predicting all-cause mortality and cardiovascular mortality, particularly under the influence of certain clinical factors including eGFR, Left Ventricular Ejection Fraction (LVEF) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) [38]. In a previous study, patients with acute decompensated HF exhibited increased GAL-3 expression levels which were associated with impaired ventricular-arterial coupling, elevated pulmonary artery pressures and severe systolic dysfunction [39]. Changes in LV structure and function associated with chronic HF are linked to serum levels of GAL-3 [40].
There was no significant difference in serum levels of GAL-3 among ESRD patients on PD either with or without LVH. However, in LVH patients, higher galaectin-3 levels were associated with higher LVEDD.
Limitation(s)
The study sample was limited. Additionally, the data presented in this study are cross-sectional and clear causation of relationship between GAL-3 and other clinical parameters cannot be determined.
Conclusion(s)
In conclusion, GAL-3 levels were reported to be a predictor of therapeutic response to ESRD patients. The GAL-3 levels in plasma are inversely related to the renal function of patients mainly without LVH. Hence, GAL-3 is not found to be a substantial clinical biomarker for the progression of cardiovascular complications in ESRD patients. Overall, these data reflect the need for further investigation of GAL-3 to HF in patients with ESRD.
BMI: Body mass index; EF: Ejection fraction; LV: Left ventricular; LVEDD: Left ventricular end diastolic diameter; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; PWT: Posterior wall thickness; TC: Total cholesterol; TGs: Triglyceride; HDL-C: High density lipoprotein cholesterol; LDL-C: Low density lipoprotein cholesterol; Non-HDL-C: Non-high density lipoprotein cholesterol and TGs/HDL-C: Triglyceride/high density lipoprotein cholesterol ratio
[1]. Gupta A, Galectin-3: Forms, Functions, and Clinical Manifestations: Editor; Gupta GS, Animal lectins: Forms, functions and clinical applications Vol III 2012 Springer-Verlag Wien10.1007/978-3-7091-1065-2 [Google Scholar] [CrossRef]
[2]. Dumic J, Dabelic S, Flogel M, Galectin-3: An open-ended storyBiochimica Et Biophysica Acta 2006 1760(4):616-35.10.1016/j.bbagen.2005.12.02016478649 [Google Scholar] [CrossRef] [PubMed]
[3]. Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, Kidney disease as a risk factor for recurrent cardiovascular disease and mortalityAm J Kidney Dis 2004 44:198-206.10.1053/j.ajkd.2004.04.02415264177 [Google Scholar] [CrossRef] [PubMed]
[4]. Chiurchiu C, Remuzzi G, Ruggenenti P, Angiotensin-converting enzyme inhibition and renal protection in nondiabetic patients: The data of the meta-analysesJ Am Soc Nephrol 2005 16(l1):S58-63.10.1681/ASN.200411096815938036 [Google Scholar] [CrossRef] [PubMed]
[5]. Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010Kidney International 2015 88(5):950-57.10.1038/ki.2015.23026221752 [Google Scholar] [CrossRef] [PubMed]
[6]. Lamb EJ, Levey AS, Stevens PE, The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolutionClin Chem 2013 59:462-65.10.1373/clinchem.2012.18425923449698 [Google Scholar] [CrossRef] [PubMed]
[7]. Yu HT, Progression of chronic renal failureArch Intern Med 2003 163(12):1417-29.10.1001/archinte.163.12.141712824091 [Google Scholar] [CrossRef] [PubMed]
[8]. Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O, Obesity and risk for chronic renal failureJ Am Soc Nephrol 2006 17(6):1695-702.10.1681/ASN.200506063816641153 [Google Scholar] [CrossRef] [PubMed]
[9]. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z, Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle?Clinical Journal of the American Society of Nephrology 2008 3(2):505-21.10.2215/CJN.0367080718184879 [Google Scholar] [CrossRef] [PubMed]
[10]. Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A, Cardiovascular disease in dialysis patientsNephrol Dial Transplant 2018 33:28-34.10.1093/ndt/gfy17430281132 [Google Scholar] [CrossRef] [PubMed]
[11]. Ronco C, The cardiorenal syndrome: Basis and common ground for a multidisciplinary patient-oriented therapyCardiorenal Med 2011 1:03-04.10.1159/00032335222258460 [Google Scholar] [CrossRef] [PubMed]
[12]. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratificationAnn Intern Med 2003 139(2):137-47.10.7326/0003-4819-139-2-200307150-0001312859163 [Google Scholar] [CrossRef] [PubMed]
[13]. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO)Kidney Int 2005 67(6):2089-100.10.1111/j.1523-1755.2005.00365.x15882252 [Google Scholar] [CrossRef] [PubMed]
[14]. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, Kidney Disease asa Risk Factor for Development of Cardiovascular Disease: A Statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and PreventionHypertension 2003 42(5):1050-65.10.1161/01.HYP.0000102971.85504.7c14604997 [Google Scholar] [CrossRef] [PubMed]
[15]. Hung SC, Lai YS, Kuo KL, Tarng DC, Volume overload and adverse outcomes in chronic kidney disease: Clinical observational and animal studiesJournal of the American Heart Association 2015 4:510.1161/JAHA.115.001918 [Google Scholar] [CrossRef]
[16]. Mukakarangwa MC, Chironda G, Bhengu B, Katende G, Adherence to hemodialysis and associated factors among end stage renal disease patients at selected nephrology units in Rwanda: A descriptive cross-sectional studyNursing Research and Practice 2018 2018:437271610.1155/2018/437271629973988 [Google Scholar] [CrossRef] [PubMed]
[17]. Yoon SN, Yang CW, Lee SH, Kim YS, Choi EJ, Chang YS, Discrepancy between solute transport rate and drain volume in CAPD patients with ascitesAdv Perit Dial 1996 12:39-42. [Google Scholar]
[18]. Cozzolino M, Galassi A, Pivari F, Ciceri P, Conte F, The cardiovascular burden in end-stage renal diseaseContrib Nephrol 2017 191:44-57.10.1159/00047925028910790 [Google Scholar] [CrossRef] [PubMed]
[19]. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE, Outcome and risk factors for left ventricular disorders in chronic uraemiaNephrology, Dialysis, Transplantation 1996 (7):1277-85.10.1093/ndt/11.7.1277 [Google Scholar] [CrossRef]
[20]. Anavekar NS, Pfeffer MA, Cardiovascular risk in chronic kidney diseaseKidney Int 2004 66(S92):S11-S5.10.1111/j.1523-1755.2004.09203.x15485401 [Google Scholar] [CrossRef] [PubMed]
[21]. Yamamoto S, Kon V, Mechanisms for increased cardiovascular disease in chronic kidney dysfunctionCurrent Opinion in Nephrology and Hypertension 2009 18(3):181-88.10.1097/MNH.0b013e328327b36019374004 [Google Scholar] [CrossRef] [PubMed]
[22]. Sahn DJ, DeMaria A, Kisslo J, Weyman A, Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurementsCirculation 1978 58:1072-83.10.1161/01.CIR.58.6.1072 [Google Scholar] [CrossRef]
[23]. Shaw LJ, Bugiardini R, Bairey Merz CN, Women and ischemic heart disease: Evolving knowledgeJ. Am. Coll. Cardiol 2009 54:1561-75.10.1016/j.jacc.2009.04.09819833255 [Google Scholar] [CrossRef] [PubMed]
[24]. Albrektsen G, Heuch I, Løchen ML, Thelle DS, Wilsgaard T, Njølstad I, Lifelong gender gap in risk of incident myocardial infarctionThe Tromsø Study JAMA Intern Med 2016 176:1673-79.10.1001/jamainternmed.2016.545127617629 [Google Scholar] [CrossRef] [PubMed]
[25]. Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the communityEur Heart J 2012 33:1734-41.10.1093/eurheartj/ehs07022507977 [Google Scholar] [CrossRef] [PubMed]
[26]. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke AssociationStroke 2014 45:1545-88.10.1161/01.str.0000442009.06663.4824503673 [Google Scholar] [CrossRef] [PubMed]
[27]. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE, Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosisNat. Rev. Cardiol 2016 13:321-32.10.1038/nrcardio.2016.4527053455 [Google Scholar] [CrossRef] [PubMed]
[28]. Levy D, Larson MG, Vasan RS, Jannel WB, Ho KK, The progression from hypertension to congestive heart failureJAMA 1996 275:1557-62.10.1001/jama.1996.035304400370348622246 [Google Scholar] [CrossRef] [PubMed]
[29]. Holmen J, Holmen TL, Tverdal A, Holmen OL, Sund ER, Midthjell K, Blood pressure changes during 22-year of follow-up in large general population- the HUNT StudyNorway BMC Cardiovasc. Disord 2016 16:9410.1186/s12872-016-0257-827176717 [Google Scholar] [CrossRef] [PubMed]
[30]. Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, Gender differences in left ventricular structure and function during antihypertensive treatment. The Losartan intervention for endpoint reduction in hypertension studyHypertension 2008 51:1109-14.10.1161/HYPERTENSIONAHA.107.10747418259011 [Google Scholar] [CrossRef] [PubMed]
[31]. Saccon F, Gatto M, Ghirardello A, Iaccarino L, Punzi L, Doria A, Role of galectin-3 in autoimmune and non-autoimmune nephropathiesAutoimmun Rev 2017 16:34-47.10.1016/j.autrev.2016.09.02327666815 [Google Scholar] [CrossRef] [PubMed]
[32]. de Oliveira FL, Gatto M, Bassi N, Luisetto R, Ghirardello A, Punzi L, Doria A, Galectin-3 in autoimmunity and autoimmune diseasesExp Biol Med (Maywood) 2015 240:1019-28.10.1177/153537021559382626142116 [Google Scholar] [CrossRef] [PubMed]
[33]. Hu Y, Yéléhé-Okouma M, Ea HK, Jouzeau JY, Reboul P, Galectin-3: A key player in arthritisJoint Bone Spine 2017 84:15-20.10.1016/j.jbspin.2016.02.02927238188 [Google Scholar] [CrossRef] [PubMed]
[34]. Meijers WC, van der Velde AR, Pascual-Figal DA, de Boer RA, Galectin-3 and post-myocardial infarction cardiac remodelingEur J Pharmacol 2015 763:115-21.10.1016/j.ejphar.2015.06.02526101067 [Google Scholar] [CrossRef] [PubMed]
[35]. Lala RI, Puschita M, Darabantiu D, Pilat L, Galectin-3 in heart failure pathology-another brick in the wall?Acta Cardiol 2015 70(3):323-31.10.1080/AC.70.3.308063726226706 [Google Scholar] [CrossRef] [PubMed]
[36]. Schindler EI, Szymanski JJ, Hock KG, Geltman EM, Scott MG, Short-and long-term biologic variability of galectin-3 and other cardiac biomarkers in patients with Stable heart failure and healthy adultsClin Chem 2016 62:360-66.10.1373/clinchem.2015.24655326546635 [Google Scholar] [CrossRef] [PubMed]
[37]. Chen A, Hou W, Zhang Y, Chen Y, He B, Prognostic value of serum galectin-3 in patients with heart failure: A meta-analysisInt J Cardiol 2015 182:168-70.10.1016/j.ijcard.2014.12.13725577755 [Google Scholar] [CrossRef] [PubMed]
[38]. Srivatsan V, George M, Shanmugam E, Utility of galectin-3 as a prognostic biomarker in heart failure: Where do we standEur J Prev Cardiol 2015 22:1096-1110.10.1177/204748731455279725268020 [Google Scholar] [CrossRef] [PubMed]
[39]. Lala RI, Darabantiu D, Pilat L, Puschita M, Galectin-3: A link between myocardial and arterial stiffening in patients with acute decompensated heart failure?Arq Bras Cardiol 2016 106:121-29.10.5935/abc.2015014926760784 [Google Scholar] [CrossRef] [PubMed]
[40]. Carrasco-Sánchez FJ, Aramburu-Bodas O, Salamanca-Bautista P, Morales-Rull JL, Galisteo-Almeda L, Páez-Rubio MI, Predictive value of serum galectin-3 levels in patients with acute heart failure with preserved ejection fractionInt J Cardiol 2013 169:177-82.10.1016/j.ijcard.2013.08.08124207066 [Google Scholar] [CrossRef] [PubMed]