Enterovesical Fistula: A Serious Complication of Crohn Disease
Atef Mejri1, Badreddine Aloui2, Khaoula Arfaoui3
1 Visceral Surgeon, Department of General Surgery, Faculty of Medicine of Tunis, Jendouba, Tunisia.
2 Visceral Surgeon, Department of General Surgery, Faculty of Medicine of Tunis, Jendouba, Tunisia.
3 Visceral Surgeon, Department of General Surgery, Faculty of Medicine of Tunis, Jendouba, Tunisia.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Atef Mejri, Jendouba Regional Hospital, Jendouba, Tunisia.
E-mail: atef.mejri@hotmail.com
Due to the anatomical proximity of the last ileal loop and the bladder, Crohn’s disease can affect the urinary tract by the formation of an Enterovesical Fistula (EVF). Although rare, it represents a complication with a potential impact on the patient’s quality of life and it is often difficult to manage. In addition to the Computed Tomographic (CT) findings, several other examinations can contribute to the diagnosis of EVF, which calls for adequate management based on disconnection of the digestive tract from the bladder and maintenance of medical treatment. Despite therapeutic advances in the context of Crohn’s disease, the EVF remains a surgical indication. The present case reported is of a successfully managed EVF which was revealed by fecaluria aiming to emphasise the clinical aspects and various diagnostic tools with a special mention of the key role of medical imaging in the diagnostic process and the surgical management.
Bladder, Inflammatory bowel disease, Surgery
Case Report
A 25-year-old male presented to the emergency room with severe abdominal pain located in the right iliac fossa evolving for five days, with no fever or vomiting and no clear aggravating or alleviating factor. He also complained of frequent urination and fecaluria that started two weeks earlier.
He was diagnosed with Crohn’s disease three years ago for which he was on imprecise medical treatment. Few months later he stopped his medication on his own and never consulted gastroenterologist. Elsewhere, he reported recurrent episodes of urinary tract infections in the past three months that were resolved with oral administration of various antibiotics.
At the time of admission, on physical examination, the patient was in general good health. The body temperature was 38°C with vital signs within normal limits: the blood pressure was 114/78 mmHg, the heart rate was 88 beats per minute, the respiratory rate was 16 breaths per minute. The abdominal examination revealed a tenderness of the right iliac fossa. No other special findings were detected.
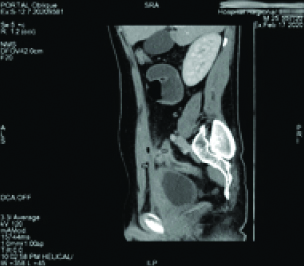
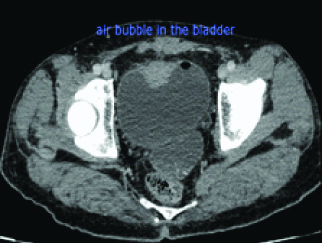
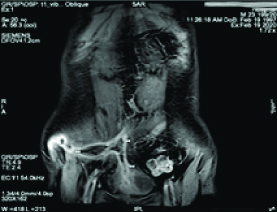
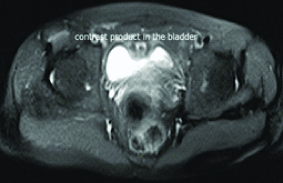
White Blood Cell (WBC) count was 7,900 cells/mm3 and the C-reactive protein level was 183 mg/dL. The urine test showed leucocyturia, but the urine culture was negative. The abdominal CT-scan showed a regular circumferential thickening of an intestinal loop located in the right iliac fossa from which a fistula took origin to a hydro aeric collection of 94x 13x 34 mm in the abdominal wall [Table/Fig-1]. A second fistula to the bladder was suspected due to the presence of an intravesical air bubble [Table/Fig-2]. An Magnetic Resonance Imaging (MRI) was performed and it showed the presence of an anteroparietal fistula with an abdominal parietal abscess measuring 85*13 mm. This abscess communicated secondarily with the bladder by an evident fistulous tract causing a thickening of its anterior wall measuring 45*12 mm [Table/Fig-3]. It also demonstrated the presence of an orally ingested product of contrast in the bladder [Table/Fig-4].
CT scan sagittal view showing the circumferential thickening of an intestinal loop located in the right iliac fossa with a fistulous tract to the abdominal wall.
A CT scan axial view showing an air bubble in the bladder.
An MRI view showing an anteroparietal fistula with an abdominal parietal abscess communicating with the bladder and the thickening of the bladder anterior wall.
MRI view showing orally ingested product of contrast in the bladder.
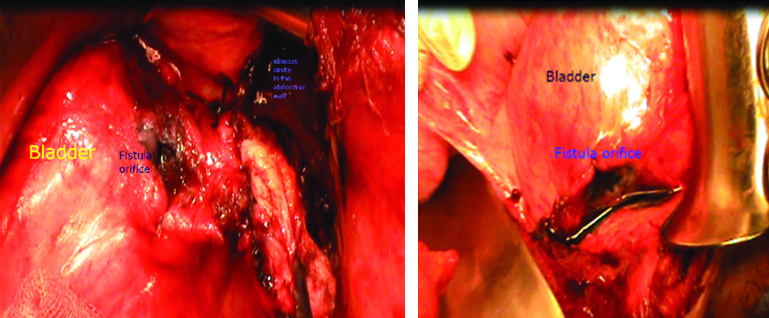
The patient underwent an open laparotomy, two days later. Intraoperatively, an entero-parietal fistula was identified with an abdominal wall abscess fistulised into the bladder (bladder dome) [Table/Fig-5,6]. Incision and drainage of the parietal abscess and entero-vesical disconnection was performed. The latter combined an ileo-caecal resection, vesicalperi-fistular area resection and bladder suture followed by an immediate restoration of continuity by a manual lateral-lateral ileo-colic anastomosis. An indwelling urethral catheter was kept in place for 10 days. Postoperative findings were uneventful. The patient was discharged from the hospital ten days after surgery and at follow-up visits, he had fully recovered.
Intraoperative view showing the fistulous bladder orifice after disconnection and the abdominal wall collection after it was drained. (Images from left to right).
Discussion
The EVF is most often the consequence of an inflammatory and infectious process of digestive origin, starting with the formation of an intra-abdominal abscess. The pus thus collected may be spontaneously fistulised either to the skin, thus creating an enterocutaneous fistula or to another segment of the intestine or to another organ, like the bladder. Usually, due to the high pressure in the intestine, the fistula takes origin from the intestine to the bladder. Diverticulitis is one of the most common aetiologies of EVF, accounting for approximately 65% to 79% of cases. Neoplasia, in second place, represent 15% to 46% of cases, followed by Crohn’s disease in 7% to 15% of cases [1]. Hardly ever, these EVF can be of radiation, post-traumatic or iatrogenic origin [1]. The clinical symptomatology is non-specific, which can lead to a diagnostic delay that may extend to several months after the onset of symptoms: pain in the right iliac fossa, change in bowel habits, burning sensation during urination, recurrent or poly microbial urinary tract infection, sediment in the urine or even epididymitis was linked to an EVF [2]. Fecaluria, as in the case of the patient and pneumaturia, although pathognomonic of entero-vesical fistulas, are only present in 50% of cases [1].
Blood tests are rarely useful for the diagnosis returning within normal limits. Urinalysis and urine culture are contributory in 85% of cases. Urinalysis, usually reveals leukocyturia and urine culture is usually positive for intestinal flora mainly E.coli. Centrifuging a urine sample can be positive for fecal debris and plant matter [3]. Radiological imaging is a crucial tool to help establish the diagnosis of an EVF, to provide a detailed description of the fistulous tract. It also gives further evaluation of the adjacent anatomy and helps identify the underlying aetiological factor and its other possible complications in the abdominal cavity, which may represent an absolute indication for early surgery. Therefore, it makes a valuable contribution in management decision-making and adjusting the surgical strategy. The plain abdominal radiograph, if conducted may show an air fluid level within the bladder that may insinuate the presence of a connection between the latter and the digestive tract [4]. Ultrasonography with a sensitivity around 71% and a specificity of 95% seems to be another helpful technique [5]. CT with a diagnostic accuracy of 90% to 100% is an optimal diagnostic modality [4]. MRI represents the modality of choice for the diagnosis of EVFs and it is more sensitive to abdominal CT to detect them. In fact, it takes the lead in precising the exact fistulous tract by showing with excellent resolution the presence of fluid inside it. The fistula is seen on T2-weighted images as a high signal tubular tract between the intestine and the bladder [3]. However, MRI has until now some limitations due to the lack of its availability and its high cost. The transit of hail, the water-soluble enema and the colonoscopy make it possible to visualise fistulas but above all to complete the lesion assessment of Crohn’s disease [3]. Another simple and inexpensive diagnosis method alternative suggests the oral intake of poppy seeds. The presence of indigestible seeds in the urine indicates the presence of an entero-vesical connection without a precise characterisation of the fistula [1,4]. Although, this procedure remains debatable. The presence of an intravesical air liquid level, the intravesical air bubble or the detection of an orally ingested product of contrast in the bladder is the radiological key signs to establish the diagnosis. The contribution of cross-sectional imaging tools is also highly considerable to draw up the lesional assessment of Crohn’s disease and to look for an associated intra-abdominal abscess, which may require emergency percutaneous drainage [4].
Management of fistula involving Crohn’s disease is challenging. It may require medical treatment with bowel rest, total parenteral nutrition, first-line immunosuppressant, corticosteroids and antibiotics in the absence of other Crohn’s disease complications [6]. Other researchers though, do not advocate the use of steroids because of the increased risk of abscess formation and active infection. In the case series reported by Zhang W et al., 5 out of 8 patients undergoing steroid treatment developed either an abdominal abscess or a persistent urinary tract infection [7]. The anti Tumour Necrosis Factor (TNF) therapy seems to be another promising option. A multicenter retrospective study carried out by Taxonera C et al., showed that 45% of patients treated with anti-TNF therapy had full remission without surgery [8]. Regardless of these encouraging results, medical treatment alone often fails to avoid surgery. It is especially reserved for minimally symptomatic patients or for those with high anaesthetic risk making them unfit for surgical interventions [4]. Yamamoto T and Keighley MR reported in their study, including 30 patients with EVF complicating Crohn’s disease, that medical treatment was effective in only four patients out of 20 who received corticosteroids with antibiotics [9]. The study by Zhang W et al., also demonstrated that concurrent Crohn’s disease complications such as abdominal abscess did not respond to medical treatment and surgery was required [7]. This matches the clinical findings in the present case report. Indeed, the presence of a quite complex fistulous tract associated with a concurrent considerable abdominal wall abscess, the young age of the patient and his medical history of lack of discipline in taking his medications, were the main reasons we decided to perform surgery. The principles of surgery are simple. They involve resection of the affected intestinal segment, immediate restoration of continuity, partial cystectomy and repair of the bladder wall, followed by the use of a Foley catheter for one week [1,4]. The closing of the bladder opening needs to be done in the simplest possible way by performing very limited excision of the inflammatory tissues followed by absorbable sutures covered by protective ommental flap [10]. The results are usually satisfying.
Conclusion(s)
EVF, although rare in patients with Crohn’s disease, are a complication requiring prompt diagnosis and treatment. The diagnosis is mainly based on clinical symptoms and indirect CT-scan results. Although its first-line treatment is medical, the management of EVF is mainly surgical consisting of intestinal resection and bladder wall repair.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 01, 2020
Manual Googling: May 01, 2020
iThenticate Software: Jul 28, 2020 (8%)
[1]. Jabbour Y, Lamzaf Y, Karmouni T, Khader K, Koutani A, Andaloussi A, Entero-Vesical fistula revealed by recurrent urinary tract infection17 août 2018 06:127-29.10.15406/unoaj.2018.06.00219 [Google Scholar] [CrossRef]
[2]. El-Haddad HM, Kassem MI, Sabry AA, Abouelfotouh A, Surgical protocol and outcome for sigmoidovesical fistula secondary to diverticular disease of the left colon: A retrospective cohort studyInt J Surg. août 2018 56:115-23.10.1016/j.ijsu.2018.05.74229902524 [Google Scholar] [CrossRef] [PubMed]
[3]. Scozzari G, Arezzo A, Morino M, Enterovesical fistulas: Diagnosis and managementTech Coloproctol 2010 14(4):293-300.10.1007/s10151-010-0602-320617353 [Google Scholar] [CrossRef] [PubMed]
[4]. Golabek T, Szymanska A, Szopinski T, Bukowczan J, Furmanek M, Powroznik J, Enterovesicalfistulae: Aetiology, imaging, and management [internet]Gastroenterology Research and Practice 2013 [cité 30 mars 2020] Disponible sur: https://www.hindawi.com/journals/grp/2013/617967/10.1155/2013/61796724348538 [Google Scholar] [CrossRef] [PubMed]
[5]. Maconi G, Sampietro GM, Parente F, Pompili G, Russo A, Cristaldi M, Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: A prospective comparative studyAm J Gastroenterol 2003 98(7):1545-55.10.1111/j.1572-0241.2003.07521.x12873576 [Google Scholar] [CrossRef] [PubMed]
[6]. Shi HY, Ng SC, The state of the art on treatment of Crohn’s diseaseJ Gastroenterol 2018 53(9):989-98.10.1007/s00535-018-1479-629980848 [Google Scholar] [CrossRef] [PubMed]
[7]. Zhang W, Zhu W, Li Y, Zuo L, Wang H, Li N, The respective role of medical and surgical therapy for enterovesical fistula in Crohn’s diseaseJ Clin Gastroenterol 2014 48(8):708-11.10.1097/MCG.000000000000004024457944 [Google Scholar] [CrossRef] [PubMed]
[8]. Taxonera C, Barreiro-de-Acosta M, Bastida G, Martinez-Gonzalez J, Merino O, García-Sánchez V, Outcomes of medical and surgical therapy for entero-urinary fistulas in Crohn’s diseaseJ Crohns Colitis 2016 10(6):657-62.10.1093/ecco-jcc/jjw01626786982 [Google Scholar] [CrossRef] [PubMed]
[9]. Yamamoto T, Keighley MR, Enterovesical fistulas complicating Crohn’s disease: Clinicopathological features and managementInt J Colorectal Dis. août 2000 15(4):211-15.10.1007/s00384000023311008720 [Google Scholar] [CrossRef] [PubMed]
[10]. Dziki Ł, Włodarczyk M, Sobolewska-Włodarczyk A, Mik M, Trzciński R, Hill AG, Is suturing of the bladder defect in benign Enterovesical fistula necessary?BMC Surg [Internet] 8 juill 2019 [cité 30 mars 2020] 19Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6615302/10.1186/s12893-019-0542-431286905 [Google Scholar] [CrossRef] [PubMed]