Introduction
With the ongoing COVID-19 pandemic, it is indispensible to minimise the risk of transmission of SARS-CoV-2 among the general population and also from positive patients to Healthcare Workers (HCW) with the help of available resources. PPE has become inevitable in this situation. PPE is defined as the materials which are designed to protect the users from transmission of infection by acting as a barrier between skin, mucous membrane and the infectious pathogens [1,2]. However, PPEs should be effectively used to avoid their shortage in future and the appropriate education of proper donning, doffing and discarding methods of PPEs, especially to health professionals is equally important to prevent people from getting exposed to contaminated PPEs.
The most abundantly used PPE currently is facemask, both in community and hospital settings. Facemasks and respirators are mainly intended to provide respiratory protection from droplets and airborne particles. Many countries across the world have made it compulsory to wear facemasks in public areas which increase the global shortage of masks [3]. As a result, few countries have prohibited the export of masks to other countries to meet their local demands. The present review focused on the current situation of shortage of masks, rational use of different types of respiratory protective devices and their reuse, mask use in laboratories and different methods of decontamination and reprocessing of the respirators. Thorough literature search of current COVID-19 pandemic and Influenza pandemic 2009 was done and various guidelines such as CDC, WHO, Public health agency of Canada were adapted for this review.
Mask Use: The Absolute Necessity
According to most of the studies and guidelines, COVID-19 is transmitted mainly by two routes, respiratory droplets and contact [2]. As the disease can be transmitted from patient during the pre-symptomatic period which is an average of 5-6 days [4], the use of masks should be considered as one of the precautionary measures to limit the transmission of viruses through respiratory droplets. However, the use of masks alone without adherence to hygienic practices such as hand hygiene and social distancing should be discouraged [4]. Inappropriate use of masks without proper care may create false sense of security to the wearers. Wearing mask would definitely complement the hygienic practices and mass masking could be considered for those essential service providers working in the community, which is likely reduce the risk of transmission [5]. MacIntyre CR and Chughtai AA conducted a systematic review of randomised controlled trial on efficacy of respiratory protective devices in different population such as HCWs, community and sick patients [6]. They found that masks and maintaining hand hygiene, both together are more protective than mask or hand hygiene alone. In HCWs, respirators were found to be effective, if worn continually during a shift, but not found to be effective if worn intermittently [6].
Significance of Cloth Masks
Shortage of PPE including N95 and medical masks has been reported worldwide, which mainly affects HCWs, among all [7].
To reserve the supply of surgical masks and N95 respirators for the frontline workers, the CDC has advised the use of cloth masks in general public, especially in the areas of significant community transmission where other important precautionary measures such as social distancing of 6 feet and hand hygiene should also be followed [8]. Those cloth masks should be regularly washed properly depending on usage frequency. The main advantage of cloth masks is that they can be made available at home using common materials. Care should be taken while removing the masks and hand hygiene has to be followed. [Table/Fig-1] shows properties of cloth mask recommended by CDC and its contraindications [9,10].
Properties of a good cloth face covering and its contraindications [9,10].
| CDC recommendation: Properties of a good cloth face covering |
|---|
It should fit snugly but comfortably against side of face Should be secured with ties or ear loops It should contain many layers of the cloth Breathing should occur without restriction It can be washed and dried without any damage
|
| Cloth masks are prohibited in the following conditions |
HCWs Children under 2 years People with breathing difficulties Unconscious patients Those who can remove masks only with assistance
|
MacIntyre CR et al., during post-influenza pandemic period in 2011, conducted a randomised trial in HCWs of 14 secondary/tertiary care hospitals in Vietnam, by comparing cloth masks with medical masks [11]. They found infection rate was lower in medical mask group compared to HCWs using cloth mask. They found that the filtration was 0% for cloth masks and also suggested that increased moisture retention capacity and ineffective cleaning and reuse of cloth masks might be responsible for this outcome.
Van der Sande M et al., during influenza pandemic preparedness in 2008, assessed the effectiveness of surgical masks, homemade masks and Filtering Facepiece 2 respirators (FFP2 respirators) respirators in healthy volunteers and simulated patients [12]. In terms of reducing risk of exposure, transmission reduction potential was twice as much in surgical masks compared to home-made masks. FFP2 masks offered 50 times more protection compared to homemade masks.
However, in view of this pandemic, general public should be strongly encouraged for using cloth mask so that N95 respirators and surgical masks can be reserved for HCWs [13]. As better respiratory protection is required for people at risk for more serious illness from COVID-19 such as older people, people with co-morbid condition, medical mask can be preferred than cloth masks in these vulnerable groups.
Medical (Surgical) Masks
Some meta-analysis studies over effectiveness of masks and respirators shows that there is no proven evidence that N95 masks are superior to surgical mask in health care providers involved in non-aerosol generating procedures against patients infected with respiratory viral infections [14,15]. N95 respirators for non-aerosol generating routine care of COVID-19 positive patients was recommended by the US CDC and European Centre for Disease and Prevention and Control [16,17], whereas, the WHO and the Public Health Agency of Canada recommend medical masks in such conditions [18,19]. In a study by Ng K et al., 35/41 HCWs who were exposed to Aerosol Generating Procedure (AGP) such as endo-tracheal intubation, extubation in COVID-19 positive patients with severe pneumonia, tested negative for COVID-19 at the end of 14 days of the exposure [20]. However, most of the guidelines strongly support the use of N95 respirators for AGPs [16-19]. Randomised controlled trial by Wong VW et al., showed use of medical masks along with other infection control practices provided effective protection against influenza [21]. Bae S et al., conducted a study in four patients with COVID-19 to evaluate the effectiveness of 100% cotton and surgical masks in blocking SARS-CoV-2 and found that both cotton masks and N95 masks were ineffective in blocking the spread of SARs-CoV -2 from the cough of COVID-19 patient [22]. Yet, the use of 3-ply surgical mask should be strongly encouraged for COVID areas where AGPs are not performed and those who are working away from the patient zone such as in the nursing station, corridor of COVID wards and for the stable quarantined person, patient attendant, non-COVID ICUs/wards and for the ambulance drivers who are not involved in patient transfer. Moreover, the effectiveness of faceshield along with surgical mask for HCWs involved in non-aerosol generating patient care should be evaluated.
N95 Respirators and Training On Use
The most frequently used PPE to protect from exposure to airborne infections is the N95 respirators. However, proper fit testing and fit checking is required to utilise the maximum protection provided by N95 respirators. Therefore, appropriate training for Healthcare Personnel (HCP) on use of these respirators is important. HCPs should be educated on limitations of its use, proper donning and doffing and methods of doing seal check. These trainings have to be done before receiving patients in COVID-19 hospital/block as a part of pandemic preparedness.
The respirators which are used by HCP for training purpose could be allowed for limited re-use of the same respirator for patient care. The N95 respirators, if used beyond the shelf life designated by the manufacturer, which is a maximum of five years from the date of manufacture [23], the quality of the material and the strap may get degraded. These expired respirators can be assigned for training purposes, but still these expired N95 respirators have a role in crisis and pandemic period [24]. Lee SA et al., tested the respiratory performance of N95 respirators and surgical masks with Sodium Chloride (NaCl) aerosols which represented bacterial and viral size range and found that N95 respirators offered 8–12 times more protection than the surgical masks [25]. Often, the HCWs experience discomfort while wearing masks and respirators due to regular use and improper wearing. Adjustment of such respiratory protective devices in the middle of the patient care activities could result in face contamination, thus leads to loss of protective benefit [26,27]. The use of N95 mask with earloop style for longer period can cause pressure injury on the skin of the ear, which makes the healthcare providers to adjust the position of the loop which inturn facilitates the transmission of infection [28]. Jiang W et al., recommended the use of plastic handle to solve this issue. After routine wearing of the mask, plastic handle has to be placed in the occipital region and from behind, each side of the handle can be hooked to the elastic bands of the mask [28].
Extended Use and Limited Reuse
Extended use of respirators is defined as using the same N95 respirators for multiple encounters with many patients without changing the respirators for each patient. This extended use is more appropriate for cohorted positive patients in hospital wards [29,30]. However, N95 respirators with visible surface contamination should not be allowed for extended use and those respirators which are involved in the AGPs should be discarded [29]. Re-use refers to removal of the respirators after patient encounter and wearing for the next patient encounter. Limited reuse was recommended during previous pandemics of respiratory pathogens [31,32]. However, if any of these practices are followed without proper hand hygiene precautions and proper fitting of mask, risk of contact transmission could be high [30,33].
Five Days Limited Re-Use of N95 Respirators (Adapted from CDC) [
34]
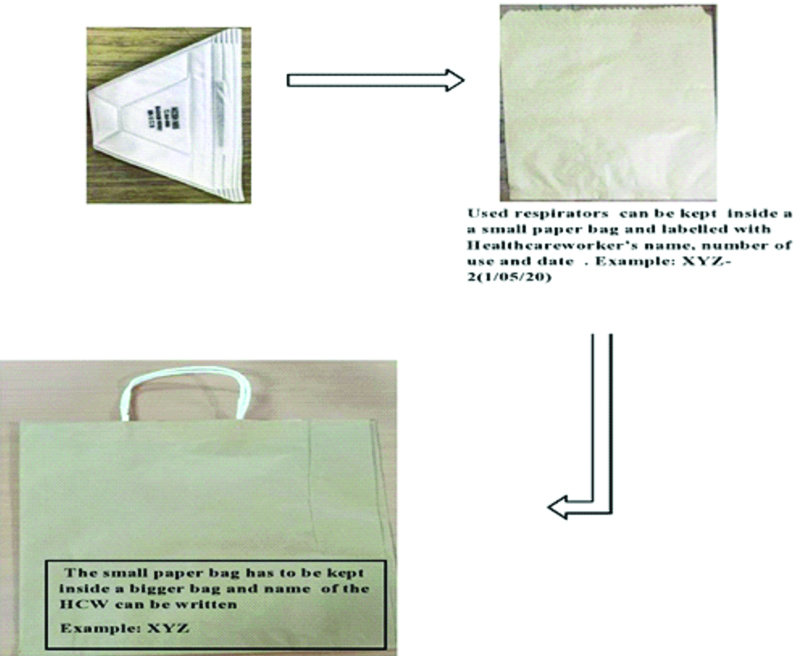
Based on the study of Van Doremalen M et al., which showed the persistance of SARS-CoV-2 on the surface of plastic, steel and cardboard for upto 72 hours [35]. CDC has made a strategy to limit the transmission of virus during the reuse of respirators by the wearer. HCPs who work in the suspected or confirmed wards may be issued with five respirators and each day one respirator will be worn and at the end of the shift it can be stored in breathable paper bag [Table/Fig-2]. Each HCPs requires five respirators and five small paper bags to keep each of the five respirators separately and one bigger paper bag in which all the five small bags can be kept. The name and the day of use has to written over the five N95 respirators (If the name of the HCW is XYZ, example: XYZ-1, XYZ-2, XYZ-3, XYZ-4, XYZ-5 has to be written over five respirators, respectively). On the first day of the shift, first mask (XYZ-1) is used and after the shift, the respirator is removed carefully without touching the filtering parts of the respirators and place it inside small paper bag and name, date and number of usage has to be written over the paper bag (XYZ-1,1/6/2020) and this smaller bag has to be kept inside a bigger paper bag and this has to be stored in a designated well aerated area. The same procedure should be followed for next five days with fresh masks and on the sixth day of the shift, the mask which was used on the first day (XYZ-1) should be re-used. This five days re-usage strategy is based on the experience of Van Doremalen M et al., who demonstrated the persistence of SARS-CoV-2 for 72 hours on various inanimate surfaces [35]. After five times of re-use, the N95 respirators should be discarded. While following this strategy, proper care has to be assured during storage and while donning the same respirators again and mask should not be taken home under any circumstances. If the shortage still exists, decontamination methods have to be adapted [34]. As the used Filtering Facepiece Respirators (FFRs) mostly contain microorganisms which is acquired from the infected patients, these are considered as potential fomites and may serve as reservoir of infection. The biological decontamination methods are mainly intended to inactivate infectious materials on the contaminated surfaces [36]. The various methods that can be applied for surface decontamination of the FFR is explained in detail further under ‘Methods of decontamination and reuse of respirators’.
Paper bags use for storage of N95 masks.
Other Alternatives to N95 Masks
The National Institute for Occupational Safety and Health (NIOSH) has approved alternative to N95 masks which offer higher or equivalent protection compared to N95 respirators include FFRs, Powered Air Purifying Respirators (PAPR), elastomeric half mask and full facepiece air purifying respirators [24,30]. Elastomeric respirators are tight fitting respirators, requires fit testing with the advantage of reuse after proper disinfection as this respirators are made of synthetic and rubber materials. PAPR has an advantage of having loose fitting hoods and does not need fit testing and also it can be reused. But, both PAPR and elastomeric respirators should be avoided in surgeries because the exhaled air may contaminate the surgical field. NIOSH approved respirators such as N99, N100, P95, P100, R99, R100 are equally protective as N95 respirators [24].
Mask Use in Laboratories
CDC has provided list of procedures which may generate aerosols in laboratories. [Table/Fig-3] shows list of AGPs done in clinical laboratories. As per CDC -guidelines for safe work practices in human and animal diagnostic laboratories in morbidity and moratality weekly report was published on January 6, 2012 [37]. Surgical masks could not be considered as respiratory PPE, personnel working in virology and mycobacteriology especially when the procedures are conducted outside the biosafety cabinet, the risk assessment should be done to determine whether N95 mask or appropriate respiratory protection is needed or not. WHO recommends use of BSL-2 laboratory and advices the use of medical masks along with hand hygiene, eye protection, gown, gloves for the laboratory technician involved in molecular testing for SARS-CoV-2 [18].
CDC: Aerosol Generating Procedures (AGP) in clinical laboratories [37].
| Aerosols are generated in the following procedures done routinely in laboratories |
|---|
| 1. Manipulation of syringes and needles (e.g., withdrawal of needles, sub-culturing positive blood culture bottles, aspiration of body fluids) |
| 2. Manipulation of inoculation loops (e.g., flaming the loops) |
| 3. Handling specimens (centrifugation, vortexing, shaking, heat fixing of smears, decanting the liquid specimens, pipetting) |
Methods of Decontamination and Reuse of Respirators
The FFRs which are disposable such as N95 respirators are generally not recommended for routine decontamination [29]. However, existing situation of shortage of PPE pushes us to ensure continuous availability of this most essential respiratory protective device. It is crucial that a decontamination procedure should retain filtration performance of the respirators, its fit characteristics and the decontamination method should be evaluated for user safety. The decontamination has to be done according to the instruction of the respirator manufacturer. A decontamination is considered to be effective, if reduction in pathogen burden is achieved without affecting the filtration efficiency and changes in the nose bridge material and strap attachment [Table/Fig-4] shows CDC recommended precautionary measures before using a decontaminated FFR [34].
CDC recommended precautionary measures before using decontaminated Filtering Facepiece Respirators (FFRs) [34].
Follow hand hygiene before and after touching the FFR and clean pair of gloves has to be used when wearing FFR Inner side of the FFR should not be touched Inspection of FFR has to be done before donning and check for the integrity of the material such as straps, nose bridge and nose foam material and should not be used, if material is not good Seal check has to be performed and if it fails, FFR should not be reused
|
The methods which can be used for decontamination of FFR include Ultraviolet Germicidal Irradiation (UVGI), Vaporous Hydrogen Peroxide (VHP), microwave generated steam, microwave steam bags, moist heat incubation and liquid hydrogen peroxide and Ethylene oxide (EtO) sterilisation [34]. Among these methods, the most promising methods for decontamination which do not affect filtration of FFRs are UVGI, VHP and moist heat according to the available studies [36]. [Table/Fig-5] shows different methods of decontamination and reprocessing of respirators and observation in related studies [38-48].
Different methods of decontamination and reprocessing of respirators and observation in related studies [38-48].
| Method | Observations in Various Studies |
|---|
| Vaporous Hydrogen Peroxide (VHP) | Passed the filtration performance According to Battelle report, straps degraded after 30 cycles [38] Fit performance was unaffected upto 20 VHP treatment cycle [39] >99.999% efficacy against Geobacillus stearothermophilus spores, T1, T7 and phi-6 bacteriophages [38-40]
|
| Ultraviolet Germicidal Irradiation (UVGI) | Passed filtration performance. Fit performance found to be 90-100% pass rate after three cycles [39,41] 99.9% efficacy against influenza A(H1NI), Avian influenza A virus (H5N1), MERS-COV, SARS-COV, influenza A (H7N9) A/Anhui/1/2013, Influenza A(H7N9) A/Shangai/1/2013 [42-44]
|
| Microwave generated steam | Passed filtration performance for 1 or 20 cycles per test [41,45] Passed fit performance for 95-100% after 3 and 20 cycles [41,45] 99.9% efficacy against H1NI influenza A/PR/8/34 [44]
|
| Ethylene oxide (EtO) | EtO did not affect the filter penetration, airflow resistance and appearance of the respirator [36,39,46] Fit performance and anti-microbial efficacy not evaluated in any of the studies Inhalation of EtO was found to be associated with neurologic dysfunction [47]
|
| Moist heat incubation | Passed fit and filtration performance [41,44,45] 99.99% efficacy against H1N1 influenza A/PR/8/34 [44]
|
| Liquid hydrogen peroxide | Passed filtration performance in six models of FFR [39,46] Fit performance and anti-microbial efficacy was not evaluated in studies
|
| Microwave steam bags | Passed filtration performance [48] Fit performance not evaluated 99.9% efficacy against MS2 bacteriophage [48]
|
Dry heat, autoclave, soap, dry microwave irradiation, 70% isopropyl alcohol have caused filter degradation and NIOSH approval has not provided [21].
Conclusion(s)
The current shortage of the PPE, predominantly the respiratory protective devices in the background of COVID-19 pandemic may continue. There is a definite need for the appropriate use of cloth masks along with precautions among the general population and rational use of surgical masks and respirators in healthcare settings, which will pave the way to efficiently manage this emergency situation. The risk assessment has to be performed based on the activity of HCWs and essential service providers. The mask use has to be optimised and prioritised approach has to considered in healthcare settings for adequate conservation of these equipment.
[1]. Centers for Disease Control and Prevention (CDC). Strategies to Optimise the Supply of PPE and Equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Accessed on May 18 [Google Scholar]
[2]. World Health Organisation. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): Interim guidance, 19 March 2020. World Health Organisation; 2020. https://apps.who.int/iris/handle/10665/331498. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
[3]. Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ, Rational use of face masks in the COVID-19 pandemicThe Lancet Respiratory Medicine 2020 8(5):434-36.10.1016/S2213-2600(20)30134-X [Google Scholar] [CrossRef]
[4]. World Health Organisation. (2020). Advice on the use of masks in the context of COVID-19: Interim guidance, 6 April 2020. World Health Organisation. https://apps.who.int/iris/handle/10665/331693. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
[5]. Cheng KK, Lam TH, Leung CC, Wearing face masks in the community during the COVID-19 pandemic: Altruism and solidarityThe Lancet 2020 Apr 10.1016/S0140-6736(20)30918-1 [Google Scholar] [CrossRef]
[6]. MacIntyre CR, Chughtai AA, A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patientsInternational Journal of Nursing Studies 2020 2020:10362910.1016/j.ijnurstu.2020.10362932512240 [Google Scholar] [CrossRef] [PubMed]
[7]. World Health Organisation. Shortage of personal protective equipment endangering health workers. published 3 March 2020 worldwide. https://www.who.int/newsroom/ [Google Scholar]
[8]. Centers for Disease Control and Prevention (CDC). Recommendation regarding the use of cloth face coverings, especially in areas of significant community-based transmission. May 16 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html [Google Scholar]
[9]. Centers for Disease Control and Prevention (CDC). How to wear cloth face coverings. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-to-wear-cloth-face-coverings.html [Google Scholar]
[10]. Centers for Disease Control and Prevention. Use of cloth face coverings to help slow the spread of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html [Google Scholar]
[11]. MacIntyre CR, Seale H, Dung TC, Hien NT, Nga PT, Chughtai AA, A cluster randomised trial of cloth masks compared with medical masks in healthcare workersBMJ open 2015 5(4):e00657710.1136/bmjopen-2014-00657725903751 [Google Scholar] [CrossRef] [PubMed]
[12]. van der Sande M, Teunis P, Sabel R, Professional and home-made face masks reduce exposure to respiratory infections among the general populationPLoS One 2008 3(7)10.1371/journal.pone.000261818612429 [Google Scholar] [CrossRef] [PubMed]
[13]. Esposito S, Principi N, Leung CC, Migliori GB, Universal use of face masks for success against COVID-19: Evidence and implications for prevention policiesEuropean Respiratory Journal 2020 Jan 1 10.1183/13993003.01260-202032350103 [Google Scholar] [CrossRef] [PubMed]
[14]. Bartoszko JJ, Farooqi MA, Alhazzani W, Loeb M, Medical masks vs N95 respirators for preventing COVID-19 in health care workers a systematic review and meta-analysis of randomised trialsInfluenza and Other Respiratory Viruses 2020 Apr 4 10.1111/irv.1274532246890 [Google Scholar] [CrossRef] [PubMed]
[15]. Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE, Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: A systematic review and meta-analysisCmaj 2016 188(8):567-74.10.1503/cmaj.15083526952529 [Google Scholar] [CrossRef] [PubMed]
[16]. Centers for Disease Control and Prevention (CDC). Interim infection prevention and control recommendations for patients with suspected or confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings March 10, 2020. https://www.cdc.gov/coronavirus/2019 ncov/infectioncontrol/controlrecommendations.html. Accessed March 17, 2020 [Google Scholar]
[17]. European Centre for Disease Prevention and Control. Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19. February 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19guidancewearingandremovingpersonalprotectiveequipmenthealthcaresettingsupdated.pdf. Accessed March 17, 2020 [Google Scholar]
[18]. World Health Organisation (WHO). Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). February 27, 2020. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO2019nCovIPCPPE_use2020.1eng.pdf. [Accessed March 17, 2020] [Google Scholar]
[19]. Public Health Agency of Canada (PHAC). Coronavirus disease (COVID-19): For health professionals. March 18, 2020. https://www.canada.ca/en/publichealth/services/diseases/2019novelcoronavirusinfection/healthprofessionals.html#i. [Accessed March 18, 2020] [Google Scholar]
[20]. Ng K, Poon BH, Kiat Puar TH, Quah JLS, Loh WJ, Wong YJ, COVID-19 and the risk to health care workers: A case reportAnn Intern Med 2020 172(11):766-67.10.7326/L20-017532176257 [Google Scholar] [CrossRef] [PubMed]
[21]. Wong VW, Cowling BJ, Aiello AE, Hand hygiene and risk of influenza virus infections in the community: A systematic review and meta-analysisEpidemiol Infect 2014 142:922-32.10.1017/S095026881400003X24572643 [Google Scholar] [CrossRef] [PubMed]
[22]. Bae S, Kim MC, Kim JY, Cha HH, Lim JS, Jung J, Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patientsAnnals of Internal Medicine 2020 Apr 6 10.7326/M20-134232251511 [Google Scholar] [CrossRef] [PubMed]
[23]. Frequently Asked Questions about the Use of Stockpiled N95 Filtering Facepiece Respirators for Protection from COVID-19 Beyond the Manufacturer-Designated Shelf Life. California Department of Public Health. Updated: March 18, 2020. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/FAQ-N95.aspx [Google Scholar]
[24]. Centers for Disease Control and Prevention (CDC). Strategies for Optimising the supply of N95 Respirators: COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html [Google Scholar]
[25]. Lee SA, Grinshpun SA, Reponen T, Respiratory performance offered by N95 respirators and surgical masks: Human subject evaluation with NaCl aerosol representing bacterial and viral particle size rangeAnnals of Occupational Hygiene 2008 52(3):177-85.10.1093/annhyg/men00518326870 [Google Scholar] [CrossRef] [PubMed]
[26]. Rebmann T, Carrico R, Wang J, Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nursesAm J Infect Control 2013 41:1218-23.10.1016/j.ajic.2013.02.01723768438 [Google Scholar] [CrossRef] [PubMed]
[27]. Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, Physical interventions to interrupt or reduce the spread of respiratory virusesCochrane Database Syst Rev 2011 (7):CD00620710.1002/14651858.CD006207.pub421735402 [Google Scholar] [CrossRef] [PubMed]
[28]. Jiang W, Cao W, Liu Q, Wear N95 mask with plastic handle reduce pressure injuryJournal of the American Academy of Dermatology 2020 Apr 10 10.1016/j.jaad.2020.04.00132283239 [Google Scholar] [CrossRef] [PubMed]
[29]. Centers for Disease Control and Prevention (CDC). Recommended guidance for extended use and limted reuse of N95 filtering facepiece respirators in healthcare settings. The National Institute for occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html [Google Scholar]
[30]. Centers for Disease Control and Prevention. Questions and answers regarding respiratory protection for infection control measures for 2009 H1N1 influenza among healthcare personnel. Retrieved October. 2009;20:2009. https://www.cdc.gov/h1n1flu/guidelines_infection_control_qa.htm [Google Scholar]
[31]. Beckman S, Materna B, Goldmacher S, Zipprich J, D’Alessandro M, Novak D, Evaluation of respiratory protection programs and practices in California hospitals during the 2009-2010 H1N1 influenza pandemicAmerican Journal of Infection Control 2013 41(11):1024-31.10.1016/j.ajic.2013.05.00623932825 [Google Scholar] [CrossRef] [PubMed]
[32]. Hines L, Rees E, Pavelchak N, Respiratory protection policies and practices among the health care workforce exposed to influenza in New York State: Evaluating emergency preparedness for the next pandemicAmerican Journal of Infection Control 2014 42(3):240-45.10.1016/j.ajic.2013.09.01324457143 [Google Scholar] [CrossRef] [PubMed]
[33]. Rebmann T, Greene LR, Hilley S, LaPointe S, Patricia Rosenbaum RN, Russell CB, APIC position paper: Extending the use and/or reusing respiratory protection in healthcare settings during disastershttp://www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/APIC_Position_Ext_the_Use_and_or_Reus_Resp_Prot_in_Hlthcare_Settings1209l.pdf [Google Scholar]
[34]. Centers for Disease Control and Prevention (CDC). Decontamination & Reuse of Filtering Facepiece Respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html [Google Scholar]
[35]. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1New England Journal of Medicine 2020 382(16):1564-67.10.1056/NEJMc200497332182409 [Google Scholar] [CrossRef] [PubMed]
[36]. Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE, Evaluation of five decontamination methods for filtering facepiece respiratorsAnnals of Occupational Hygiene 2009 53(8):815-27.10.1093/annhyg/mep070 [Google Scholar] [CrossRef]
[37]. Miller JM, Astles R, Baszler T, Chapin K, Carey R, Garcia L, Guidelines for safe work practices in human and animal medical diagnostic laboratoriesMMWR Surveill Summ 2012 6(61):01-02. [Google Scholar]
[38]. Battelle. Final Report for the Bioquell Hydrogen peroxide Vapor (HPV) Decontamination for Reuse of N95 Respirator. 2016. Available: https://www.fda.gov/emergency-preparedness-and-response/mcm-regulatory-science/investigating-decontamination-and-reuse-respirators-public-health-emergenciesexternal icon [Google Scholar]
[39]. Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR, Shaffer RE, Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respiratorsJournal of Engineered Fibers and Fabrics 2010 5(4):15589250100050040510.1177/155892501000500405 [Google Scholar] [CrossRef]
[40]. Kenney P, Chan BK, Kortright K, Cintron M, Havill N, Russi M, Hydrogen peroxide vapor sterilisation of N95 respirators for reuseMed Rxiv 2020 Jan 1 10.1101/2020.03.24.20041087 [Google Scholar] [CrossRef]
[41]. Bergman MS, Viscusi DJ, Palmiero AJ, Powell JB, Shaffer RE, Impact of three cycles of decontamination treatments on filtering facepiece respirator fitJournal of the International Society of Respiratory Protection 2011 28(1):4810.1080/15459624.2011.58592721732856 [Google Scholar] [CrossRef] [PubMed]
[42]. Fisher EM, Shaffer RE, A method to determine the available UV C dose for the decontamination of filtering facepiece respiratorsJournal of Applied Microbiology 2011 110(1):287-95.10.1111/j.1365-2672.2010.04881.x21054699 [Google Scholar] [CrossRef] [PubMed]
[43]. Mills D, Harnish DA, Lawrence C, Sandoval-Powers M, Heimbuch BK, Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respiratorsAmerican Journal of Infection Control 2018 46(7):e49-55.10.1016/j.ajic.2018.02.01829678452 [Google Scholar] [CrossRef] [PubMed]
[44]. Heimbuch BK, Wallace WH, Kinney K, Lumley AE, Wu CY, Woo MH, A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and dropletsAmerican Journal of Infection Control 2011 39(1):e01-09.10.1016/j.ajic.2010.07.00421145624 [Google Scholar] [CrossRef] [PubMed]
[45]. Viscusi DJ, Bergman MS, Novak DA, Faulkner KA, Palmiero A, Powell J, Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning easeJournal of Occupational and Environmental Hygiene 2011 8(7):426-36.10.1080/15459624.2011.58592721732856 [Google Scholar] [CrossRef] [PubMed]
[46]. Viscusi DJ, King WP, Shaffer RE, Effect of decontamination on the filtration efficiency of two filtering facepiece respirator modelsJournal-International Society for Respiratory Protection 2007 24(3/4):93 [Google Scholar]
[47]. Rutala WA, Weber DJ, Guideline for disinfection and sterilisation in healthcare facilities 2008 https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ [Google Scholar]
[48]. Fisher EM, Williams JL, Shaffer RE, Evaluation of microwave steam bags for the decontamination of filtering facepiece respiratorsPLoS One 2011 6(4)10.1371/journal.pone.001858521525995 [Google Scholar] [CrossRef] [PubMed]