Introduction
Though target sedation was achieved with Midazolam and Dexmedetomidine, Dexmedetomidine has demonstrated the lesser complications and shorter duration of stay in Intensive Care Unit (ICU). Most of the studies are reported from high income countries. The studies on Midazolam and Dexmedetomidine use in mechanically ventilated children are scanty in low-middle income regions.
Aim
To compare the efficacy of Midazolam and Dexmedetomidine for sedation in mechanically ventilated children.
Materials and Methods
This prospective observational cohort study was conducted in academic hospital Paediatric Intensive Care Unit (PICU) from March 2015 to June 2016. Children aged less than 13 years mechanically ventilated for more than 24-hour and received sedative with either infusion of Midazolam or Dexmedetomidine without loading dose were involved. Patients with unstable haemodynamic throughout PICU stay and expired within 24-hour and incomplete medical data were excluded. Intermittent Fentanyl/Morphine was used as when needed as per treating team decisions. Sedation assessment was performed with Ramsey sedation scale (RSS, target=3-4 out of 6), Tracheal suctioning score and PICU sedation score. The primary outcome was “percentage of time with target sedation” till extubation. The secondary outcome was the cumulative dose of sedation used, the need for rescue sedation and the rate of complications, organ dysfunction {by Sequential Organ Failure Assessment (SOFA) score and Paediatric Logistic Organ Dysfunction (PELOD) score} and the length of stay in ventilation, PICU and mortality.
Results
A total of 115 patients (Midazolam-group, n=63 and Dexmedetomidine-group, n=52) were enrolled. The median age was 12 months (IQR 8-30). Mean (±SD) PRISM-III score was 11.3±7.2. About 54.8% were ventilated for respiratory pathology, followed by CNS pathology (25.2%) and sepsis (10.4%). Mean (±SD) percentage of the duration of proper sedation was not significantly different in Midazolam-group (83.4±15.6) and Dexmedetomidine-group (81.4±17) (p=0.510). The cumulative dose (microgram per kg) requirement was higher in Midazolam-group {median (IQR) 12.2 (9.8-17.1) vs. 9.6 (5-15.3); p=0.019)}.
No difference was note in need for “rescue dose of sedation” per patient {median (IQR) 1 (0-2) vs. 1 (0-2)}, rate of complications (bradycardia 9.5% vs. 1.9%; hypotension 9.5% vs. 5.8%). No difference was noted in organ dysfunction score {mean difference, 95% CI; SOFA score: -0.2 (-1.6 to 1.33); p=0.808 and PeLOD score: 1.3 (-1.5 to 4.1); p=0.364}, duration of ventilation (median, IQR 2.7 (2-3.3) vs. 2.0 (1.5-3.1) days and mortality (20.6% vs. 21.2%). PICU stay was significantly lower in Midazolam-group (median, IQR 3 days, 1-5 vs. 5 days, 4-6; p=<0.001).
Conclusion
Midazolam and Dexmedetomidine were associated with similar target sedation with a comparable rate of complications in mechanically ventilated children. However, Midazolam required a higher cumulative dose to achieve target sedation.
Complications, Paediatric intensive care, Sedation
Introduction
Sedation and analgesia are an integral part of the management of critically ill children on mechanical ventilation as these aid to reduce anxiety, agitation and pain [1,2]. It also prevents patient-ventilator asynchrony, accidental extubation and displacement of invasive vascular devices. The most commonly used drugs for sedation in critical care units belong to γ-Aminobutyric Acid (GABA) receptor agonists (Propofol and Midazolam) and opioids (Morphine, Fentanyl) [1,3]. These drugs have been associated with tolerance, physical dependency, paradoxical agitation, withdrawal, inconsistent sedation and respiratory depression [3]. The available alternative sedatives are Dexmedetomidine, Triclofos Sodium, Chloral Hydrate, and Phenobarbital [4].
Dexmedetomidine is an alpha-2 adrenergic agonist and active dextro isomer of medetomidine. It exhibits specificity for alpha-2 versus alpha-1 of 1600:1. By activation of postsynaptic alpha-2 adrenergic receptors in the central nervous system, it produces sedation, anxiolysis and analgesia. By stimulation of parasympathetic outflow produces sedation and anxiolysis. Inhibition of sympathetic outflow results in a decrease in heart rate and blood pressure [1].
Midazolam is an imidazobenzodiazepine. It produces sedation, anxiolysis and anterograde amnesia effects but no analgesic effect. It is a water-soluble acidic preparation, at plasma pH converts into a unionised form that crosses the Blood-Brain Barrier (BBB) rapidly; it has the shortest elimination half-time of the benzodiazepine group [1,4,5]. After prolonged administration, sedation effects may persist for 48 hours even after discontinuation of the agent, and it’s called ‘Midazolam infusion syndrome.’ Benzodiazepines and opioids can cause neuroapoptosis and neurodevelopmental abnormalities [5]. However, Dexmedetomidine has shown potential neuroprotective properties, including the prevention of induced neuroapoptosis [5].
The available evidence, including controlled studies, suggest that time to achieve target sedation was similar in both Midazolam and Dexmedetomidine. The Dexmedetomidine has demonstrated the 20% less chance of delirium, fewer rescue bolus doses of morphine, a lesser frequency of assessment and shorter stay of ICU. These studies are mainly reported in high-income countries [1,4]. Practice guidelines and available literature have been identified as the need for more research comparing the effectiveness of Dexmedetomidine and Midazolam in PICU patients [4], particularly low-middle income countries where constrained in the availability of resource against the demand of services.
Thus, the study aimed to compare the Dexmedetomidine and Midazolam in children requiring mechanical ventilation.
Materials and Methods
Study Design
This was a prospective observational cohort study conducted in a division of Paediatric critical unit of a tertiary care academic hospital from March 2015 to June 2016. The PICU is 19 bedded and accepts both medical and surgical patients. The institutional ethics committee approved the study with a waiver of written consent (JIP/IEC/2015/11/509).
Inclusion criteria: Children aged ≤12-year mechanically ventilated for more than 24-hour and received a continuous infusion of sedation either Dexmedetomidine (0.20 to 1.2 microgram per kg per hour) or Midazolam (0.06 to 6 microgram per kg per minutes) with intermittent Fentanyl/Morphine, as needed.
Exclusion criteria:
Patients with unstable haemodynamics throughout PICU stay (Mean arterial blood pressure ≤ fifth percentile for age, sex, length/height with ≥ two vasoactive therapy supports and/or requiring ongoing resuscitation).
Died within 24-hour of PICU admission.
Incomplete/missing data.
Procedure and Data Collection
The decision regarding the starting and titration of the sedative agent was made as per the treating physicians’ discretion. Patient were divided into two groups (Midazolam and Dexmedetomidine groups) based on type medication received for sedation throughout stay in PICU. Sedation assessment performed with Ramsey Sedation Scale (RSS score 1 to 6; higher the score deeper the sedation), PICU sedation score (1 to 4; higher the score deeper the sedation) and Tracheal suctioning score (1 to 5; higher the score deeper the sedation) [1]. The quality of sedation was assessed every hourly (more frequently if needed) with RSS score, and sedation drug was titrated to achieve the RSS score of 3 to 4 out of 6. During the suctioning of endotracheal tube tracheal suctioning score was used and sedation drug was titrated if tracheal suctioning scores of 1 to 2 out of 5.
Overall, sedation status was assessed by PICU sedation score every four hours (more frequently if needed) with a target score of 2 out of 4. The sedation score used in the study is given in [Table/Fig-1].
Sedation score used in the study [1].
| Ramsay Sedation Score (RSS)1 - Anxious, agitated, restless2 - Eyes open, cooperative, oriented, tranquil3 - Responds (opens eyes) only to command, light touch, normal tone of voice4 - Brisk response to light glabellar tap or loud noise/voice5 - Sluggish response to light glabellar tap or loud noise/voice6 - No response to light glabellar tap or loud noise/voice |
| Tracheal Suctioning Score1 - Patient is restless or distressed when not disturbed2 - Patient is awake and moving, but not distressed if left alone3 - Movement only with nursing care, major limb movement/distress with tracheal suctioning4 - Cough, grimace or minor limb movement with suctioning5 - No response to tracheal suctioning |
| PICU Sedation Score1 - Awake, alert2 - Occasionally drowsy, easy to arouse3 - Frequently drowsy, easy to arouse4 - Somnolent |
Every day morning 6 am sedation holiday was given and assessed for ready to extubation as per unit protocol. The patients’ vitals, demographic data, level of sedation, blood investigations details, need for rescue sedation, a dosage of sedation required, length of ventilation, PICU stay, and adverse events of sedation drug were noted in structured proforma.
Outcome measures: The primary outcome was “percentage of time with target sedation” till extubation. The secondary outcomes were the cumulative dose of sedation used, the need for rescue doses of fentanyl/morphine, and the rate of complications, organ dysfunction (by SOFA score and PELOD score) and the length of stay in mechanical ventilation, PICU and mortality.
Statistical Analysis
Infusion of Midazolam and Fentanyl (Morphine) was the most frequently used sedation in mechanically ventilated children in PICU. Taking the probability to use of Dexmedetomidine is 20% with the degree of variation 8% with 95% confidence interval and degree of precision 8%, the minimum sample size of 96 was needed [1,3]. With an attrition rate of 10% for incomplete or missing data, the final sample size was 110. At end of study 63 patients in Midazolam group and 52 patients in Dexmedetomidine group was enrolled.
Percentage of time within the target sedation range during the sedation infusion was calculated by dividing the total time that the patients remained within the target sedation range (RSS score 3 to 4) by the amount of time the patient remained in the infusion of sedation drug, multiplied by 100.
The normality of data was checked with the Kolmogorov-Smirnov Z test. The continuous data between the two groups were compared by Student’s t-test if by normally distributed or Mann-Whitney U test if skewed data. The proportion was compared with the Chi-square test (or Fisher’s-exact test if cell frequency is small). Kaplan-Meier and log-rank tests were used for the analysis of ‘time to event’ data followed by Cox regression analysis done to adjust for prespecified baseline factors (age, sex and PRISM-III score). The relative risk/hazard ratio with 95% confidence interval was calculated as appropriate. All tests two-tailed, and p-value of less than 0.05 was considered significant. SPSS 20.0 software (SPSS Inc. Chicago, Illinois) and Epi Info™ 7 (7.0.9.7, CDC) was used for data analysis. The statistician was blinded for treatment group classification until the preparation of the first draft of the manuscript.
Results
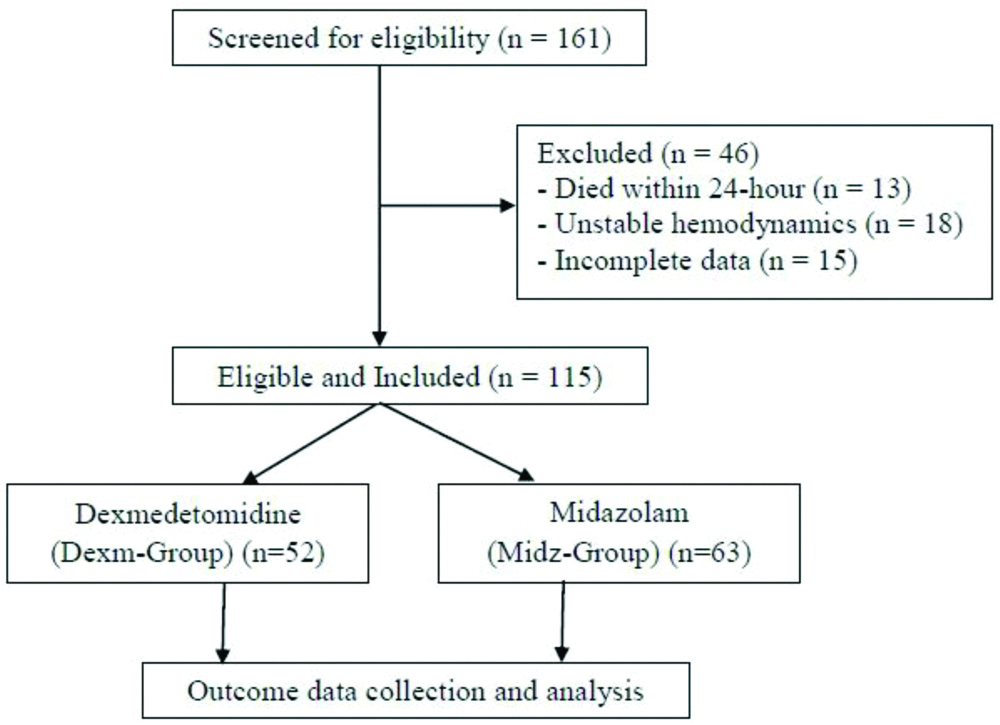
After the screening of 161 patients, 115 eligible patients were enrolled (Midazolam-group, n=63 and Dexmedetomidine-group, n=52) [Table/Fig-2]. The baseline characteristics and clinical data of the study participants are given in [Table/Fig-3] and comparable except the age of the study participant. Half of the patients (54.8%) were ventilated for respiratory pathology, followed by central nervous system pathology (25.2%) and sepsis (10.4%). Most of the patients belonged to the younger age group (median age 12-month, interquartile range 8 to 30-month).
Baseline characteristics and clinical data of the study participants.
| Variable | All patients (n=115) | Midazolam group (n=63) | Dexmedetomidine group (n=52) | p-value |
|---|
| Age in months, median (IQR) | 12 (8-30) | 19 (10-37) | 11 (6-19) | 0.010* |
| Male: Female, n (%) | 68 (59): 47 (41) | 33 (52.4): 30 (47.6) | 35 (67.3): 17 (32.7) | 0.105† |
| Weight, kg | 10.05±5.24 | 11.18±5.14 | 8.68±5.09 | 0.010‡ |
| Weight, z score | 1.43±1.11 | 1.52±1.01 | 1.31±1.21 | 0.300‡ |
| Height, cm | 75.18±16.79 | 76.06±15.95 | 74.12±17.86 | 0.538‡ |
| Height, z score | 0.93±1.02 | 0.84±0.95 | 1.04±1.08 | 0.302‡ |
| PRISM-III score | 11.3±7.2 | 11±7.3 | 11.7±7 | 0.616‡ |
| Hyperglycaemia, n (%) | 12 (10.4) | 5 (7.9) | 7 (13.5) | 0.426† |
| Hypoglycaemia, n (%) | 11 (9.6) | 9 (14.3) | 2 (3.8) | 0.058§ |
| Primary diagnosis||, n (%) |
| Pneumonia | 45 (39) | 23 (36.5) | 22 (42.3) | 0.526† |
| Bronchiolitis | 18 (15.7) | 9 (14.2) | 9 (17.3) | 0.657† |
| Acute CNS infections | 9 (7.8) | 5 (8) | 4 (7.7) | 1.000§ |
| Tuberculous meningitis | 6 (5.2) | 4 (6.3) | 2 (3.8) | 0.688§ |
| Status epilepticus | 14 (12.2) | 10 (15.9) | 4 (7.7) | 0.254§ |
| Severe sepsis | 12 (10.4) | 7 (11.1) | 5 (9.6) | 0.794† |
| Haemolytic uremic syndrome | 3 (2.6) | 2 (3.2) | 1 (2) | 1.000§ |
| Others | 8 (7.1) | 3 (4.8) | 5 (9.6) | 0.465§ |
| Ventilator parameters |
| -Max PIP, cm H2O | 20.1±4.3 | 20±3.8 | 20.4±4.8 | 0.550‡ |
| -Max PEEP, cm H2O | 6.3±2.1 | 6.6±1.9 | 6±2.3 | 0.119‡ |
| -Max Fio2, % | 61.6±21.8 | 63±20.6 | 60±23.3 | 0.460‡ |
| -Lowest compliance, mL per cm H2O | 4.3±1.7 | 4.1±1.6 | 4.5±1.9 | 0.159‡ |
All the data are presented as mean (SD) unless otherwise mentioned. IQR: Interquartile range; SD: Standard deviation; PRISM: Paediatric risk of mortality; PIP: Peak inspiratory pressure; PEEP: Positive end-expiratory pressure; FiO2: Fraction of inspired oxygen. ||Few cases had overlapped with another system of involvement. *Mann Whitney U test; †Chi-square test; ‡Student t-test; §Fisher’s-exact test
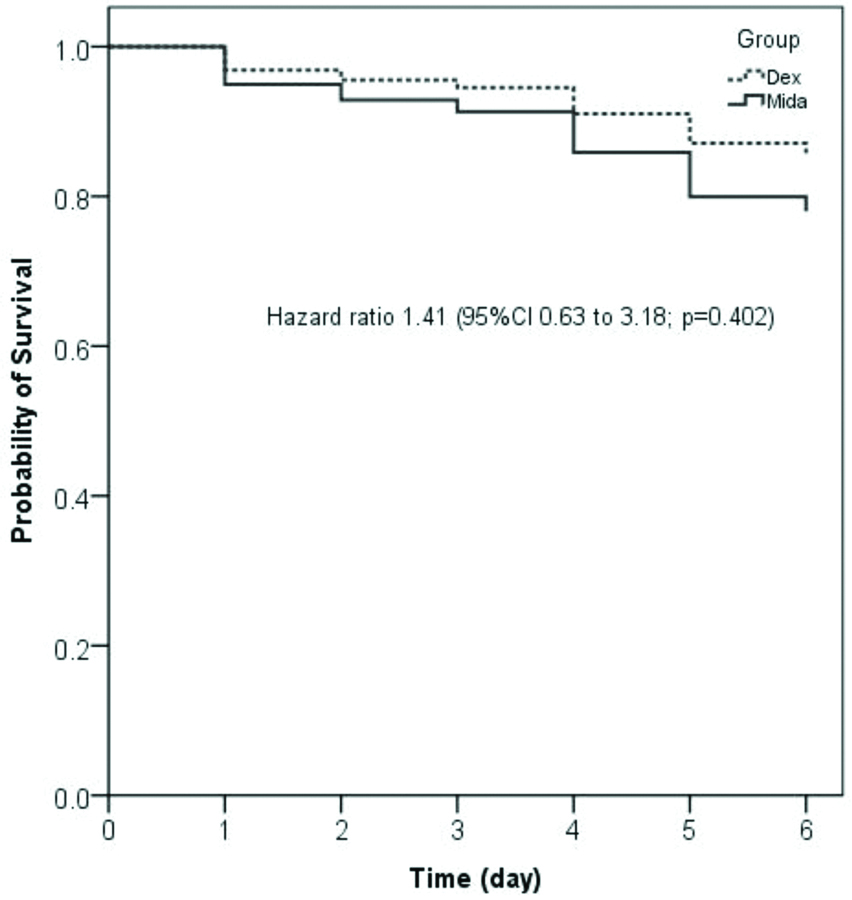
The outcomes of the study participant are given in [Table/Fig-4]. The primary outcome of the study (percentage of time with target sedation) was similar between Midazolam-group and Dexmedetomidine-group (mean±SD, 83.4±15.6 vs. 81.4±17; p=0.510). There was no difference in under sedation and over sedation between the two groups. The mean (SD) sedation assessment scores (RSS, Tracheal suction, and PICU sedation score) during the infusion of sedation drug in PCIU stay found that there was no significant difference between the two groups. The cumulative dose of sedation drug required to achieve the target sedation was higher in Midazolam-group as compared to Dexmedetomidine-group {median (IQR), 12.2 (9.8-17.1) vs. 9.6 (5-15.3) microgram per kg; p=0.019}. The need for rescue doses of sedation drugs, organ dysfunctions, rate of complications and length of mechanical ventilation was similar between the two study groups. However, median (IQR) PICU stays was lower in Midazolam-group as compared to Dexmedetomidine-group 3 (1-5) vs. 5 (4-6) calendar days; p=<0.001} [Table/Fig-4]. There was no significant difference noted in mortality between the two study groups {20.6% vs. 21.2%; relative risk 1.02, 95% CI (0.62 to 1.66); p=0.946 and hazard ratio 1.41, 95% CI (0.63 to 3.18); p=0.402} [Table/Fig-4,5].
| Variable | Midazolam group (n=63) | Dexmedetomidine group (n=52) | Mean difference/Relative risk (95% confidence interval) | p-value |
|---|
| Percentage of time with target sedation | 83.4±15.6 | 81.4±17 | -2.0 (-8.0 to 4.0) | 0.510* |
| Percentage of time with under sedation | 13±13.4 | 9±7.2 | -4.1 (-8.1 to 0.03) | 0.052* |
| Percentage of time with over sedation | 4±12.8 | 9.7±19.4 | 5.8 (-0.2 to 11.8) | 0.058* |
| Ramsay sedation score during sedation period | 3.7±0.3 | 3.6±0.5 | -0.09 (-0.2 to 0.06) | 0.214* |
| Tracheal suction score during sedation period | 3.6±1.8 | 3.5±0.4 | -0.2 (-0.7 to 0.3) | 0.536* |
| PICU sedation score during sedation period | 3.4±0.3 | 3.1±0.4 | -0.3 (-0.5 to -0.2) | <0.001* |
| Cumulative dose, median (IQR) (microgram per kg) | 12.2 (9.8-17.1) | 9.6 (5-15.3) | - | 0.019† |
| Need for rescue sedation doses per patient, median (IQR) | 1 (0-2) | 1 (0-2) | - | 0.799† |
| Complications, n (%) | | | | |
| Bradycardia | 6 (9.5) | 1 (1.9) | 3.31 (0.53 to 20.51) | 0.126‡ |
| Hypotension | 6 (9.5) | 3 (5.8) | 1.39 (0.54 to 3.57) | 0.509‡ |
| SOFA score Day-1 | 5.8±4.4 | 4.4±29 | -1.3 (-2.7 to 0.1) | 0.075* |
| SOFA score During PICU stay | 4.4±3.2 | 4.3±4.3 | -0.2 (-1.6 to 1.3) | 0.808* |
| PELOD score Day-1 | 11.3±8.7 | 11±8.8 | -0.3 (-3.6 to 2.9) | 0.845* |
| PELOD score During PICU stay | 7±5.7 | 8.2±9 | 1.3 (-1.5 to 4.1) | 0.364* |
| Mechanical ventilation, days, median (IQR) | 2.7 (2-3.3) | 2.0 (1.5-3.1) | - | 0.193† |
| PICU stay, days, median (IQR) | 3 (1-5) | 5 (4-6) | - | <0.001† |
| Mortality n (%) | 13 (20.6) | 11 (21.2) | 1.02 (0.62 to 1.66) | 0.946§ |
All the data are presented as mean (SD) unless otherwise mentioned. IQR: Interquartile range; SD: Standard deviation; PICU: Paediatric intensive care unit; SOFA: Sequential organ failure assessment; PELOD: Paediatric logistic organ dysfunction. Over-sedation was defined as Ramsay sedation score of 5 or 6 and under-sedation was defined as Ramsay sedation score of 1 or 2. *Student t-test; †Mann-Whitney U test; ‡Fisher’s-exact test; §Chi-square test
Survival curve showing mortality in the study groups.
Discussion
This observational cohort study found that target sedation in mechanically ventilated children by Midazolam and Dexmedetomidine was achieved with a comparable rate of complications. There was no difference in length of ventilation, organ dysfunction and mortality except lower PICU stay in Midazolam-group. However, Midazolam group patients required a higher cumulative dose as compared with Dexmedetomidine group.
Studies found that continuous infusion of sedation was an independent predictor of prolonged mechanical ventilation and led to more extended ICU and hospital stay [4,6]. Hence, the implementation of daily interruption of sedation has led to the shorter length of stay in mechanical ventilation, ICU and hospital [7,8]. The daily interruption of sedation in PICU was associated with shorter duration of ventilation, PICU stays, a lower dose of sedation and lower cost of therapy [9,10]. In the present study, continuous infusion with daily interruption of sedation might be a reason for a similar primary outcome measure in two study groups. Continuous sedation with daily interruption might provide advantages of titratable, easy to maintain target sedation and avoid sudden agitation though the risk of deep sedation, which can be avoided by frequent monitoring of sedation level. The controlled study by Tobias JD et al., found that Dexmedetomidine at a dose of 0.25 micrograms per kg per hour provides a similar sedation level by Midazolam at a dose of 0.22 mg per kg per hour [1]. At 0.5 microgram per kg per hour provided superior sedation as compared to Midazolam by decreased need for supplemental Morphine and decreased in the number of inadequate sedation. Further, Dexmedetomidine was less effective in an infant less than 12-month in 5 of 6 patients [1]. In this study, the dose used was within the acceptable dose range and target sedation was able to achieve sedation in the infant by both study groups.
Tobias JD and Berkenbosch JW (2004), reported similar sedation assessment score (RSS, tracheal suction and PICU sedation score) and bispectral index by Midazolam and two different doses of Dexmedetomidine in mechanically ventilated children [1]. The results are similar to the current study, but bispectral index was not monitored in this study. This study found that the need for rescue/supplemental sedation was similar in both the groups. Other studies reported that despite having equivalent Ramsay score, the patient receiving loading dose followed by infusion of Dexmedetomidine had a significant decrease in supplemental sedation [1,11,12]. The loading dose followed by infusion might be the reason behind the need for decreased supplemental sedation, but loading dose was not used in this study.
The current study reported a similar cardiovascular complication rate in Midazolam and Dexmedetomidine groups (bradycardia 9.5% vs. 1.9% and hypotension 9.5% vs. 5.8%). As with currently using many medications in PICU, there is a potential for side effects of the cardiovascular system with Dexmedetomidine. Other studies, reported up to 30% of haemodynamic complications (either bradycardia or hypotension) by use of Dexmedetomidine for sedation purpose [11,13,14]. These studies have used loading dose followed by infusion of Dexmedetomidine. In this study, loading dose was not used. Hence, continuous infusion without loading dose can achieve desirable sedation without the need for an additional supplemental dose of sedation and a lower rate of adverse effects on haemodynamic variables in mechanically ventilated children.
Limitation(s)
The study has both limitations and strength. First, this was a single-center and not a controlled study. Second, the hypothesis and data collection were conceived prospectively with reasonable sample size. Third, in this study, bispectral index was not used for assessment of the level of sedation. Fourth, the adverse effects of Dexmedetomidine on respiratory function were not assessed because of the given nature of study participants (mechanically ventilated patients). However, previous studies have reported the feasibility of weaning and tracheal extubation while patients are receiving Dexmedetomidine [1,15,16]. This study found that the length of mechanical ventilation was similar between the two study groups. Fifth, in this study assessment of withdrawal syndrome and delirium was not done. The current study paw the path for future controlled studies in PICU including sub-population of patients in particularly low-middle income countries. The preferably multicentric studies are needed.
Conclusion(s)
The study concludes that the infusion of Midazolam and Dexmedetomidine was associated with similar target sedation with comparable complications in mechanically ventilated children. However, Midazolam required a higher cumulative dose to achieve target sedation as compared with Dexmedetomidine. The continuous infusion without a loading dose and with a daily interrupted protocol of sedation might be a reason for the achievement of similar target sedation and a feasible sedation approach in the PICU.
All the data are presented as mean (SD) unless otherwise mentioned. IQR: Interquartile range; SD: Standard deviation; PRISM: Paediatric risk of mortality; PIP: Peak inspiratory pressure; PEEP: Positive end-expiratory pressure; FiO2: Fraction of inspired oxygen. ||Few cases had overlapped with another system of involvement. *Mann Whitney U test; †Chi-square test; ‡Student t-test; §Fisher’s-exact test
All the data are presented as mean (SD) unless otherwise mentioned. IQR: Interquartile range; SD: Standard deviation; PICU: Paediatric intensive care unit; SOFA: Sequential organ failure assessment; PELOD: Paediatric logistic organ dysfunction. Over-sedation was defined as Ramsay sedation score of 5 or 6 and under-sedation was defined as Ramsay sedation score of 1 or 2. *Student t-test; †Mann-Whitney U test; ‡Fisher’s-exact test; §Chi-square test
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 22, 2020
Manual Googling: Jun 01, 2020
iThenticate Software: Jul 24, 2020 (14%)
[1]. Tobias JD, Berkenbosch JW, Sedation during mechanical ventilation in infants and children: Dexmedetomidine versus midazolamSouth Med J 2004 97:451-55.10.1097/00007611-200405000-0000715180019 [Google Scholar] [CrossRef] [PubMed]
[2]. O’Connor M, Bucknall T, Manias E, Sedation management in Australian and New Zealand intensive care units: Doctors’ and nurses’ practices and opinionsAm J Crit Care 2010 19:285-95.10.4037/ajcc200954119770414 [Google Scholar] [CrossRef] [PubMed]
[3]. Tobias JD, Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unitCrit Care Med 2000 28:2122-32.10.1097/00003246-200006000-0007910890677 [Google Scholar] [CrossRef] [PubMed]
[4]. Koizumi T, Kurosawa H, Survey of analgesia and sedation in pediatric intensive care units in JapanPediatr Int 2020 62:535-41.10.1111/ped.1413931910495 [Google Scholar] [CrossRef] [PubMed]
[5]. Lei S, Lu P, Lu Y, Zheng J, Li W, Wang N, Dexmedetomidine alleviates neurogenesis damage following neonatal midazolam exposure in rats through JNK and P38 MAPK pathwaysACS Chem Neurosci 2020 11:579-91.10.1021/acschemneuro.9b0061131999428 [Google Scholar] [CrossRef] [PubMed]
[6]. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G, The use of continuous i.v. sedation is associated with prolongation of mechanical ventilationChest 1998 114:541-48.10.1378/chest.114.2.5419726743 [Google Scholar] [CrossRef] [PubMed]
[7]. Kress JP, Pohlman AS, O’Connor MF, Hall JB, Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilationN Engl J Med 2000 342:1471-77.10.1056/NEJM20000518342200210816184 [Google Scholar] [CrossRef] [PubMed]
[8]. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trialLancet 2008 371:126-34.10.1016/S0140-6736(08)60105-1 [Google Scholar] [CrossRef]
[9]. Gupta K, Gupta VK, Jayashree M, Singhi S, Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated childrenPediatr Crit Care Med 2012 13:131-35.10.1097/PCC.0b013e31820aba4821283046 [Google Scholar] [CrossRef] [PubMed]
[10]. Verlaat CW, Heesen GP, Vet NJ, de Hoog M, van der Hoeven JG, Kox M, Randomized controlled trial of daily interruption of sedatives in critically ill childrenPaediatr Anaesth 2014 24:151-56.10.1111/pan.1224523980693 [Google Scholar] [CrossRef] [PubMed]
[11]. Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unitAnaesthesia 1999 54:1136-42.10.1046/j.1365-2044.1999.01114.x10594409 [Google Scholar] [CrossRef] [PubMed]
[12]. Martin E, Ramsay G, Mantz J, Sum-Ping ST, The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unitJ Intensive Care Med 2003 18:29-41.10.1177/088506660223912215189665 [Google Scholar] [CrossRef] [PubMed]
[13]. Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgeryAnesth Analg 2000 90:834-39.10.1213/00000539-200004000-0001110735784 [Google Scholar] [CrossRef] [PubMed]
[14]. Peden CJ, Cloote AH, Stratford N, Prys-Roberts C, The effect of intravenous dexmedetomidine premedication on the dose requirement of propofol to induce loss of consciousness in patients receiving alfentanilAnaesthesia 2001 56:408-13.10.1046/j.1365-2044.2001.01553.x11350323 [Google Scholar] [CrossRef] [PubMed]
[15]. Belleville JP, Ward DS, Bloor BC, Maze M, Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rateAnesthesiology 1992 77:1125-33.10.1097/00000542-199212000-000131361310 [Google Scholar] [CrossRef] [PubMed]
[16]. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ, Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusionsAnesth Analg 2000 90:699-705.10.1097/00000539-200003000-0003510702460 [Google Scholar] [CrossRef] [PubMed]