Diphtheria is a vaccine preventable communicable acute infectious disease of upper respiratory tract caused by C. diphtheriae [1]. C. diphtheria causes disease by production of an extremely potent cytotoxic exotoxin that destroys host cells by inhibiting protein synthesis. C. diphtheriae occurs in four biotypes: gravis, intermedius, belfanti and Mitis. C. diphtheria gravis causes the most severe form of disease due to high potency of toxin [2]. It is uncommon in developed countries like United States but endemic in India [3]. Diphtheria mainly affects children aged 1 year to 5 years. A shift in the age wise incidence of the disease from pre-school to school age (5 to 15 years) has been observed with more cases now reported among adults [4]. Incidence reported in India during 1980 was about 39,231 with DTP3 coverage of 58% and it declined to 15,685 in 1985 after the successful implementation of Universal Immunisation Programme with DTP3 coverage of 100%. Diphtheria re-emerged after successful control with vaccination from 1,326 in 1997 to 8,465 cases in 2004 due to drop in immunisation coverage from 93% in 1997 to 63% in 2004 which could be attributed to lack of awareness and misconception. Incidence reported in India during 2017 was 5,293 and in 2018 was 8,788 [5].
According to a study done by Murhekar M, India accounted for majority of diphtheria cases reported globally [6]. As per Central Bureau of Health Intelligence (CBHI) data, during 2005-2014, India reported 41,672 cases with 897 deaths. Ten Indian states which include then Andhra Pradesh (now Telangana and Andhra Pradesh), Assam, Delhi, Gujarat, Haryana, Karnataka, Nagaland, Maharashtra, Rajasthan and West Bengal accounted for 84% cases reported [6]. Delayed diagnosis of the disease leads to spread of infection in the community and causes increased morbidity and mortality in the affected individuals. To reduce the delay, an early attempt for microbiological diagnosis of diphtheria should be done as it is crucial and complimentary to clinical diagnosis, as diphtheria is often confused with other conditions, such as severe streptococcal sore throat, Vincent’s angina or glandular fever [7,8].
The need of the present study is mainly to know the incidence of disease in different age groups to emphasise the strict implementation of immunisation and prevalence of different biotypes causing the disease to know the severity of the disease and its course as gravis biotype causes severe disease compared to other biotypes. Thus, the aim of this study was to assess the incidence of clinical diphtheria in different age groups, gender predisposition, role and limitations of microscopy in diagnosis and prevalent biotypes of C. diphtheriae causing the disease.
Materials and Methods
The present study was a prospective study conducted at a tertiary care centre, Sir Ronald Ross Institute of Tropical and Communicable Diseases, Hyderabad, Telangana, India, during July 2017 to December 2017 after getting approval from Institutional Ethics Committee (Reg no: 1611900112D) of Osmania Medical College, Hyderabad, affiliated to KNR University of Health Sciences, Warangal, Telangana, India. Samples were taken from 300 clinically probable [9] cases of diphtheria. Based on the average of the incidence and prevalence of the disease in the institute in previous years sample size was calculated using the formula n=4pq/d2.
Inclusion criteria: All types of patients were included irrespective of immunisation status. Patients of both sexes in between 2 and 60 years of age, admitted with complaints of fever, sore throat, patch on tonsils, difficulty in swallowing, difficulty in breathing, bull neck. Informed consent was obtained from all the patients included in the study.
Exclusion criteria:
1) Patients less than 2 years and more than 60 years of age.
2) Patients who were unwilling to participate in the study.
Methodology
At the time of admission, two throat swabs were collected from probable cases of diphtheria. First swab was inoculated into LSS [Table/Fig-1] and with the same swab smears were prepared for Albert stain. After six hours, smear was prepared from LSS, stained with Albert stain [10] and compared with the microscopical examination of the smear from direct throat swab (first swab from which smears were made directly). Blood Agar (BA), Potassium Tellurite Agar (PTA) plates were used for primary isolation [Table/Fig-2,3] on which the second swab was inoculated for culture, which was considered as confirmatory method of diagnosis. Typical colonies from BA, PTA plates were subjected to microscopic examination for Klebs-Loeffler Bacillus (KLB) by Gram stain and Albert stain after 24 and 48 hours of incubation at 37°C. Identification till species level was done using standard biochemical tests and antimicrobial susceptibility testing was done using Kirby- Bauer disc diffusion method by using 0.5 McFarland standard inoculum prepared from direct colony suspension on BA with antibiotic discs, Penicillin (PEN), Amoxyclav (AMC), Erythromycin (E), Azithromycin (AZM), Ciprofloxacin (CIP) and Ceftriaxone (CTR) [11].
Loeffler’s Serum Slope (LSS).

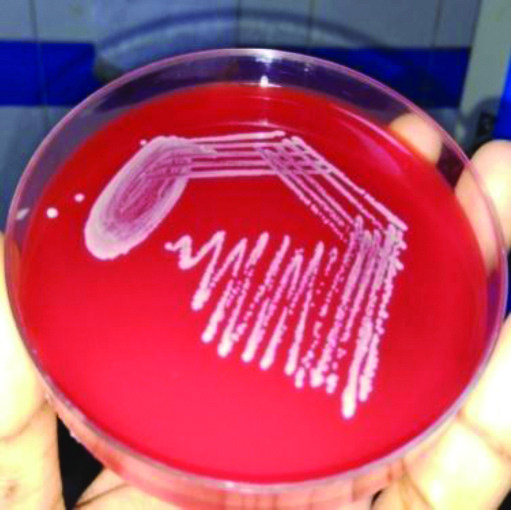
C.diphtheriae colonies on BA.
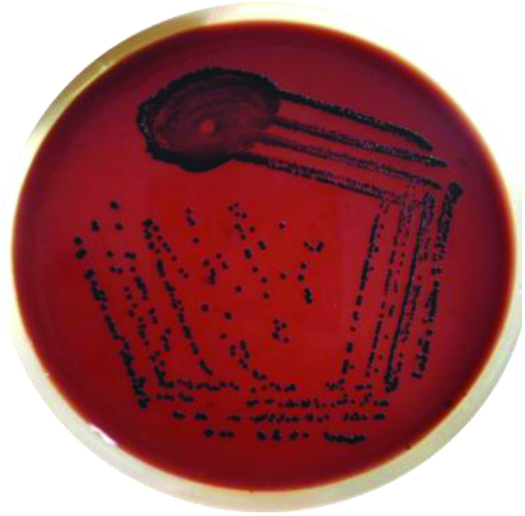
C.diphtheriae colonies on PTA.
Statistical Analysis
The results were analysed after entering the values in MS Excel for percentages and comparisons.
Results
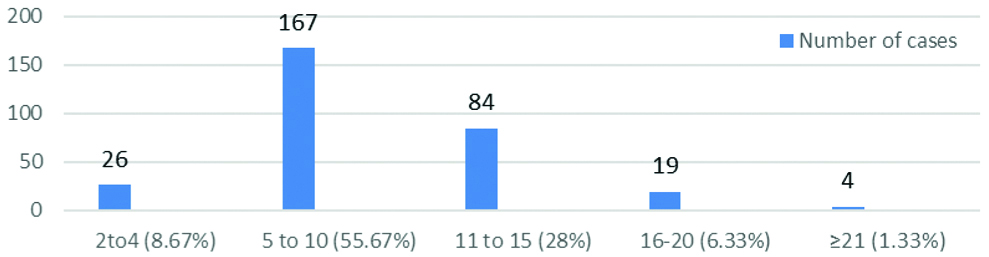
Total number of clinically probable cases of diphtheria were 300. The highest number of cases, 167 (55.67%) were in the age group 5-10 years and the lowest number of 4 (1.33%) were aged ≥21 years [Table/Fig-4]. Clinically, probable cases were more seen in females, 166 (55.33%) than males 134 (44.67%). Cases diagnosed presumptively by microscopical examination of the smear from direct throat swab were 9 (3%), whereas confirmed cases by culture were 48 (16%). Comparison of direct smear and culture of clinically probable cases was done in which true positive cases (smear and culture +) were 6, true negative (smear and culture -) were 249, false positive (smear + and culture-) were 3 and false negative (smear – and culture +) were 42 [Table/Fig-5]. Sensitivity (12.5%) was very low than Specificity (98.81%) for direct smear of KLB. Culture should be advised, even if the direct smear is negative.
Age distribution of clinically probable cases of diphtheria.
Comparison of direct smear and culture (confirmatory method of diagnosis) in clinical cases of diphtheria.
| Culture | Smear for Klebs-Loeffler Bacillus (KLB) |
|---|
| Positive (9) | Negative (291) |
|---|
| Positive (48) | 6 | 42 |
| Negative (252) | 3 | 249 |
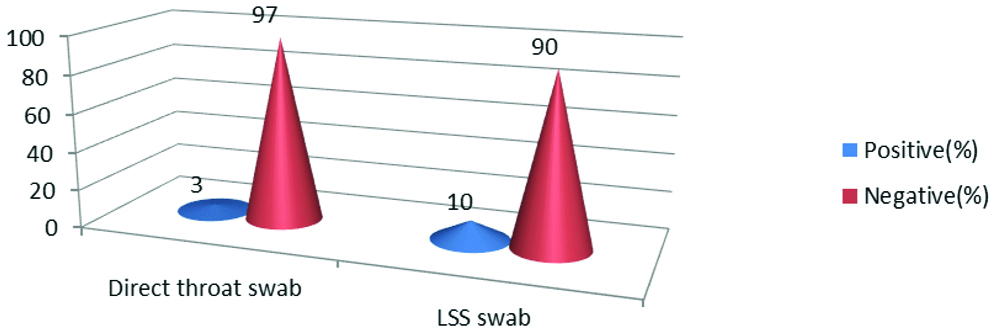
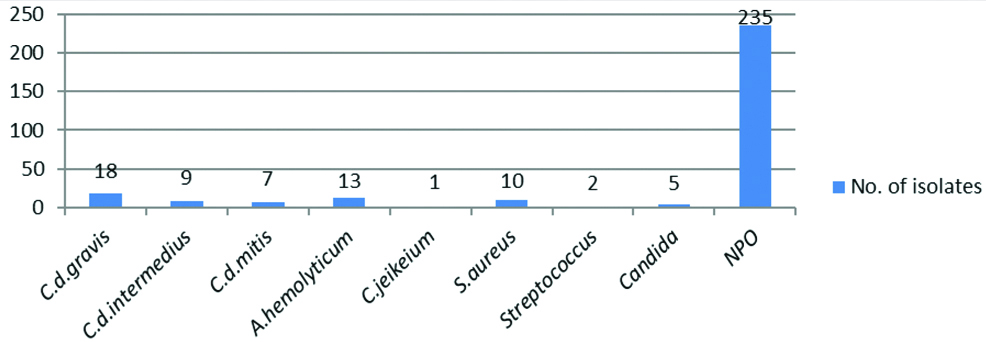
Presumptive diagnosis by microscopic examination of smears from direct throat swab and LSS in which throat swab was inoculated showed 7% increase in positivity from LSS swabs [Table/Fig-6]. Organisms isolated from throat swab samples collected from probable clinical cases of diphtheria were C. diphtheriae biotypes 34/300 (11.33%), Arcanobacterium haemolyticum 13/300 (4.33%), Corynebacterium jeikeium 1/300 (0.33%), Staphylococcus aureus 10/300 (3.33%), Streptococcus spp 2/300 (0.67%), Candida spp 5/300 (1.67%) and No Pathogenic Organism (NPO) in 78.34% cases. Out of 48/300 isolates of culture confirmed diphtheria cases C. diphtheriae biotypes gravis were 18/48 (37.5%), intermedius 9/48 (18.75%) and mitis 7/48 (14.59%). Other Corynebacterium spp, C.jeikeium were 1/48 (2.08%), and Coryneform Gram positive bacilli, A.haemolyticum were 13/48 (27.08%). C.diphtheriae gravis isolation was higher when compared to other biotypes followed by A.haemolyticum [Table/Fig-7].
Comparison of presumptive diagnosis by microscopic examination of smears from direct throat swab and LSS.
Organisms isolated from throat swab samples collected from clinical cases of diphtheria.
All the 48 culture isolates were sensitive to all antibiotics tested, i.e., PEN, AMC, E, AZM, CIP and CTR discs with no resistance pattern.
Discussion
Diphtheria can lead to significant morbidity and mortality because of its severe critical complications such as obstructive airway disease, myocarditis, polyneuritis, cranial nerve palsies, and secondary pneumonia, if not diagnosed and treated promptly [4]. In the present study, out of 300 probable cases of diphtheria incidence in 5-20 years age group was 90% showing a shift of the disease from below 5 years i.e., preschool children to older children i.e., 5-20 years, adolescents and young adults which might be due to waning antibody titres in older children, adolescents and young adults due to improper coverage of booster dosage of vaccination. Similar results were seen in study done by Mohan DG et al., Saikia L et al., and Basavaraja GV et al., [Table/Fig-8] [4,12,13], whereas in contrast, Bhagat S et al., reported high incidence of disease in 1-5 years age group [7]. Study done by Bitragunta S et al., showed higher incidence among 5-19 years age group and in females [14].
Shift in incidence of disease from preschool to school age [4,12,13].
| Study | Incidence in age groups (%) |
|---|
| Pre-school (<5 year) | School age (5-15 year) |
|---|
| Saikia L et al., (2010) [12] | 0 | 100 |
| Basavaraja GV et al., (2016) [13] | 25.8 | 74.1 |
| Mohan DG et al., (2018), [4] | NA | 84.6 (5-14 y) |
| Present study | 8.67 | 83.67 (79.66 in 5-14 y) |
Female predominance was seen with 55.33% incidence in this study in clinically probable cases of diphtheria similar to studies done by Meera M and Rajarao M [1] with 60%, and Basavaraja GV et al., with 51.6% incidence whereas in study conducted by Sharma NC et al., male predominance was seen with 61.5% incidence [13,15].
In the present study, direct smear positivity for KLB was 3%, similar results were seen in Mohan DG et al., with 4.04% whereas study conducted by Basavaraja GV et al., showed smear positivity rate of 32.2%, where the study was conducted in 31 patients [4,13]. Culture positivity was 16% similar to studies done by Meera M and Rajarao M with 18.8%, Basavaraja GV et al., with 16.1%, culture positivity [1,13], whereas Mohan DG et al., showed 26.26% [4]. Bhagat S et al., 23%, Elantamilan D et al., showed 25% culture positivity [7,16]. Sensitivity and specificity of direct smear for KLB taking culture as confirmatory method for diagnosis of disease was 12.5% and 98.81%, respectively in this study, similar to study done by Meera M and Rajarao M where sensitivity was 21.12% and specificity was 100% in contrast Mohan DG et al., showed 100% sensitivity and 94.52% specificity [1,4]. The cause for cases showing negative smear and positive culture might be due to low sensitivity of direct smear examination, objective difference of the observer who reports the direct smear and also improper collection of sample in patients who were unable to open their mouth due to pain. These causes might be the limitations of microscopical examination of direct smear, limiting its role in diagnosis. According to Brooks R, the diagnosis of diphtheria should not be attempted, solely based on the microscopical examination of a direct smear, since both false positive and false negative results are likely to occur [17].
In the present study, when microscopy from direct throat swab and swab inoculated in LSS were compared, it showed a 7% increase in positivity rate of microscopy by inoculating the swab in LSS. In this study of all Corynebacterium isolates, the biotypes of C. diphtheriae were 70.84%, Corynebacterium other than diphtheria (C.jeikeium) were 2.08%, and Coryneform Gram positive bacilli (A.haemolyticum) were 27.08%. Similar results were seen in Meera M and Rajarao M with all variants of C. diphtheriae accounting to 72% cases where as other than diphtheriae species isolated were 28% [1]. Sharma NC et al., showed 98.9% isolation of C.diphtheriae variants and 1.1% isolation of Corynebacterium other than diphtheria [15]. Mohan DG et al., showed 100% isolation of all C. diphtheriae variants [4]. In the present study, C.diphtheriae gravis isolation was higher when compared to other biotypes with 52.94% of total C.diphtheriae isolates whereas intermedius was 26.47% and mitis was 20.59%. In study done by Sharma NC et al., C.diphtheriae intermedius isolation was higher when compared to other biotypes with 95.45% of total C.diphtheriae isolates whereas gravis was 3.41% and mitis was 1.14% [15]. In cases where the isolated strain was gravis showed severe form of clinical disease, and longer duration for recovery whereas milder forms of disease was seen in other biotypes of C.diphtheriae and other spp of Corynebacterium. Similar results were seen in study done by Meera M and Rajarao M [1].
No resistance among the 48 isolates was found for the antibiotics tested i.e., PEN, AMC, E, AZM, CIP and CTR discs. All the antibiotics tested were sensitive. PEN drug can be used effectively for treatment purposes, recommended dose being 20,000-100,000 units for serious cases, half of the dose being given intravenously. In cases where PEN allergy is seen, Erythromycin can be recommended which is more active than PEN in treating carriers [10]. In a study conducted by Sharma NC et al., 54 isolates were tested for 17 drugs-Amoxyclav (AMC, 30 mcg), Ampicillin (AMP, 10 mcg) Ampicillin+ Sulbactam (AMS, 20 mcg), Azithromycin (AZM, 15 mcg) Cephotaxime (CTX, 30 mcg), Cefoxitin (CX, 30 mcg), Ceftriaxone (CTR, 30 mcg), Cefuroxime Sodium (CXM, 30 mcg), Chloramphenicol (C, 30 mcg), Ciprofloxacin (CIP, 5 mcg), Clindamycin (CD, 2 mcg) Doxycycline (DC, 30 mcg), Erythromycin (E, 15 mcg), Gentamycin (GET, 10 mcg), Penicillin (P, 10 mcg), Tetracycline (TE, 30 mcg) Cotrimoxazole (COT, 12.5/23.75 mcg) in which 45 (83.3%) isolates showed resistance to Co-trimoxazole, 10 (18.5%) to Ampicillin, 1 (1.85%) was resistant to CIP and no isolate showed multi drug resistance and all 54 isolates were sensitive to all other 14 drugs [15].
The present study infers that more number of clinically probable cases was seen in 5-10 years age group with female predominance. For diagnostic purposes culture should be considered as confirmatory method, and sole relying on microscopy leads to false negative results.
Limitation(s)
Antimicrobial Susceptibility Testing was done by disc diffusion, though the standard method is micro broth dilution method.
Conclusion(s)
Diphtheria is still a life threatening disease causing clinical illness. A shift in the age wise incidence of the disease from pre-school to school age has been observed with more cases reported, which calls for intensive implementation, monitoring of immunisation and need for introduction of booster doses of vaccination in the immunisation schedule above the age of 5 years. Culture should be considered as confirmatory method to diagnose the clinically probable cases of diphtheria. C. diphtheriae biotype gravis was the highly prevalent strain isolated.