Diabetes mellitus is the fourth major cause of mortality among non-communicable diseases with 1.5 million deaths globally as per the WHO report in 2012 [1]. Adherence to healthy lifestyle was shown to be beneficial by minimising carotid intima media thickness and HbA1c levels among men [2]. Early age of onset of diabetes, elevated HbA1c levels and history of severe hypoglycaemia were shown to increase risk for cognitive impairment and dementia in type 2 diabetes mellitus [3]. Iron bioavailability was reported to have an inverse association with body mass index while reduced physical work capacity was associated with lower transferrin saturation in adolescents with nonalcoholic fatty liver disease [4].
Elderly subjects with type 2 diabetes mellitus were recently reported to have iron deficiency to the extent of 30.7% making them prone for microcytic anaemia [5]. Treatment of iron deficiency was reported to reduce HbA1c levels with simultaneous improvement in mean corpuscular volume and mean corpuscular haemoglobin [6]. Iron deficiency anaemia was shown to be associated with higher HbA1c levels even in non-diabetic population thus creating impediment to diagnostic or therapeutic decision based on HbA1c levels [7,8]. HbA1c elevation in iron deficiency is more prominent in subjects with fasting blood glucose in the range of 100-126 mg/dL than those with normal glucose levels [9].
Transferrin is a systemic iron transporter. An invitro study demonstrated that glycated transferrin has lesser iron binding capacity than the non glycated transferrin [10]. Lys534 and Lys206 residues of transferrin were reported to be more vulnerable to glycation [10]. Another invitro study indicated that glycation impairs Fe3+ binding and thus affecting Fe3+- transferrin isoform distribution depending on the glucose concentration [11]. Higher transferrin glycation was observed in diabetes with more pronounced elevation in type 1 diabetes [12]. The iron ions in the glycated transferrin molecule were reported to be loosely bound to the protein and thus impairing the function of transferrin as an iron binding protein [13].
In the current study, the aim was to explore the association of transferrin glycation with altered iron metabolism in uncontrolled diabetes.
Materials and Methods
A total of 136 subjects comprising of 50 non-diabetic, 53 controlled diabetics and 33 uncontrolled diabetic subjects were enrolled for this cross-sectional study, conducted at the out-patient unit of Nizam’s Institute of Medical Sciences, Hyderabad, India. The recruitment took place between the period of January, 2019 to August, 2019. The data collection and analysis were performed during the period of September 2019 to December 2019.
Based on the data of Christy AL et al., the HbA1c levels in subjects with iron deficiency anaemia and non-anaemic controls were 6.87±1.7% and 5.65±0.69%, respectively [9]. Using the mean values of the groups and common standard deviation, the sample size estimated was 30 (15 samples per group) to obtain 90% power with a type I alpha error of 0.05.
There were 74 men and 62 women in the study. The study protocol was approved (EC/2164/2018) by the Institutional Ethical Committee. Written informed consent was obtained from all the study participants.
The inclusion criteria were: i) the subjects in the age group of 25 to 65 year; ii) fasting blood glucose and HbA1c levels being performed most recently to distinguish between diabetic vs. non-diabetic. The exclusion criteria were: i) subjects who didn’t gave consent to participate in the study; ii) subjects with any inflammatory disease or cancer.
Blood samples were collected in K2EDTA for HbA1c assay and plain vacutainers for biochemical analysis. Serum was collected from plain vacutainers by centrifugation at 2000 × g for 10 minutes at room temperature.
Biochemical Analysis
Glycosylated haemoglobin
HbA1c levels were estimated by ion-exchange High-Performance Liquid Chromatography (HPLC) using Bio-Rad D-10TM Dual Program. The samples were automatically diluted on the D-10 and injected into the analytical cartridge. The resolution of haemoglobin was performed by D10 using a gradient mobile phase of increasing ionic strength and the absorbance was measured at the λmax of 415 nm. An exponentially modified Gaussian algorithm was employed for the calculation of A1c area to exclude the areas of interfering peaks of the labile A1c and carbamylated Hb.
Serum iron, TIBC, ferritin and transferrin assays were performed on Roche Cobas c501 Chemistry Analyser using kits manufactured by Roche Diagnostics, USA.
Serum Iron
Serum iron levels were estimated by FerroZine method. Under acidic conditions, iron is liberated from transferring in the form of Fe+3, which undergoes reduction in the presence of ascorbate to form Fe+2 ions. The Fe+2 ions form a coloured complex with Ferrozine. The colour intensity is directly proportional to the iron concentration. It was determined by monitoring the increase in absorbance at 552 nm.
Total Iron Binding Capacity
The Unsaturated Iron-Binding Capacity (UIBC) in human serum was measured by FerroZine spectrophotometrically at a λmax of 552 nm. The sum of serum iron and UIBC was computed as TIBC. The percentage saturation of transferrin was calculated using the following formula: (serum iron × 100)/TIBC.
Ferritin
Serum ferritin levels were estimated by using Particle Enhanced Immunoturbididimetric Assay. Human ferritin agglutinates with latex particles coated with anti-ferritin antibodies. The precipitate is determined turbidimetrically at 552 nm.
Transferrin
Transferrin levels in serum were estimated by Immunoturbidimetric assay at λmax of 340 nm by precipitating it using a specific antiserum.
Tranferrrin Glycation by Isoelectric Focusing
The serum samples from uncontrolled diabetic patients were stored at -20°C till use and kept on ice when used. A 1 mm native IEF gel with a pH gradient of 3.0-7.0 was used to run the samples along with a marker (1 μL control sample+1 μL neuraminidase enzyme, 37°C for 1 h) and a non-diabetic control sample. Selective silver staining of transferrin was performed using Polyclonal Rabbit Anti- human Transferrin.
Statistical Analysis
Simple regression analysis followed by Chi-square test was carried out to study changes in markers of iron metabolism across different measurable ranges of HbA1c. The unpaired student t-test was performed to find out the differences in HbA1c in iron deficiency vs. iron sufficiency; and also to compare biochemical markers based on presence or absence of transferrin glycation.
Results
The study cohort (74 men and 62 women in the age group of 25-65 years) was divided into three groups based on HbA1c levels: <6.0% (Group I)- Non-diabetic, 6.1-8.0% (Group II)- Controlled diabetic and >8.0% (Group III)- Uncontrolled diabetic. The mean age of the subjects was 47±12 years. Iron, % saturation, TIBC and ferritin were compared across these three groups. Serum iron <50 ng/dL and % saturation <14 were considered as deficient. TIBC >400 μg/dL and Ferritin >250 ng/mL were considered as elevated.
As shown in [Table/Fig-1], the incidence of iron deficiency in non-diabetic subjects (Group I) was 20%. There was a significant increase in iron deficiency in diabetic groups with Group II (41.5%) and Group III (60.6%). Simple logistic regression analysis revealed statistically significant association between HbA1c vs. iron deficiency (p=0.02).
Biomarkers of iron metabolism across different ranges of HbA1c.
| HbA1c | <6.0% (n=50) (Non-diabetic) | 6.4-8.0% (n=53) (Controlled diabetic) | >8.0% (n=33) (Uncontrolled diabetic) | χ2 | p-value |
|---|
| Deficient | Normal | Deficient | Normal | Deficient | Normal | | |
|---|
| Iron | 10 (20.0%) | 40 (80.0%) | 22 (41.5%) | 31 (58.5%) | 20 (60.6%) | 13 (39.4%) | 5.39 | 0.02 |
| % Saturation | 3 (6.0%) | 47 (94.0%) | 17 (32.1%) | 36 (67.9%) | 19 (57.6%) | 14 (42.4%) | 11.9 | 0.0006 |
| Elevated | Normal | Elevated | Normal | Elevated | Normal | | |
| Ferritin | 5 (10.0%) | 45 (90.0%) | 2 (3.8%) | 51 (96.2%) | 8 (24.2%) | 25 (75.8%) | 0.2 | 0.65 |
| TIBC | 3 (6.0%) | 47 (94.0%) | 8 (15.1%) | 45 (84.9%) | 10 (30.3%) | 23 (69.7%) | 6.27 | 0.01 |
Simple logistic regression followed by Chi-square test were performed to explore the trend with changing HbA1c levels.
The frequencies of % saturation deficiency were 6.0%, 32.1% and 57.6%, respectively in Group I, Group II and Group III. Simple logistic regression analysis revealed statistically significant association between HbA1c vs. deficient percentage saturation (p=0.0006).
Elevated ferritin levels were observed in 10.0% non-diabetic subjects. In Group II and Group III, ferritin was elevated in 3.8% and 24.2% subjects, respectively. Simple logistic regression analysis showed no statistically significant association between HbA1c and Ferritin elevation (p=0.65).
The frequency of TIBC elevation was highest in Group III (26.1%) followed by Group II (15.1%) with non-diabetic subjects having the lowest frequency (6.0%). Simple logistic regression analysis showed statistically significant association between HbA1c and TIBC elevation (p=0.01).
In order to assess the impact of iron deficiency in modulating HbA1c levels, in each group data was segregated based on iron deficiency vs. iron sufficiency. As shown in [Table/Fig-2], iron deficiency did not affect the HbA1c levels in Group I and Group II, while iron deficiency was associated with statistically significant increase in HbA1c in Group III, (p=0.03).
Iron deficiency across different ranges of HbA1c. The unpaired student t-test was performed to explore the differences in HbA1c in iron deficiency vs. iron sufficiency.
| Groups | HbA1c % (mean±SD) | p-value |
|---|
| Iron deficiency | Iron sufficiency |
|---|
| Group I | 5.46±0.38 (n=10) | 5.38±0.49 (n=40) | 0.63 |
| Group II | 6.82±0.63 (n=33) | 6.80±0.44 (n=20) | 0.92 |
| Group III | 9.90±1.10 (n=20) | 8.90±0.98 (n=13) | 0.03* |
SD: Standard deviation
As shown in [Table/Fig-3], isoelectric focusing was performed to assess transferrin glycation in a few representative samples with uncontrolled diabetes as serum transferrin levels were at the lower quartile in uncontrolled diabetes (HbA1c levels of 13.4±1.2%). Isoelectric focusing differentiate five isoforms of transferrin; tetrasialo, trisialo, disialo, monosialo and asialo isoforms. In non-diabetic patients all the forms of transferrin isoforms are observed. In uncontrolled diabetes, only tetrasialo form was observed.
Transferrin isoelectric focusing.
Lane 1-6 are uncontrolled diabetic subjects while Lane 7-8 are controls. Lane 1, 2 and 5 showed increased glycation and showed higher levels of TIBC and ferritin and lower levels of serum transferrin.
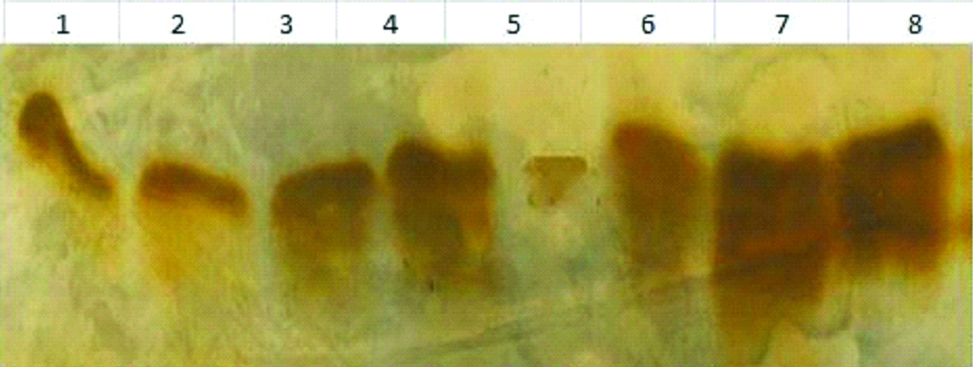
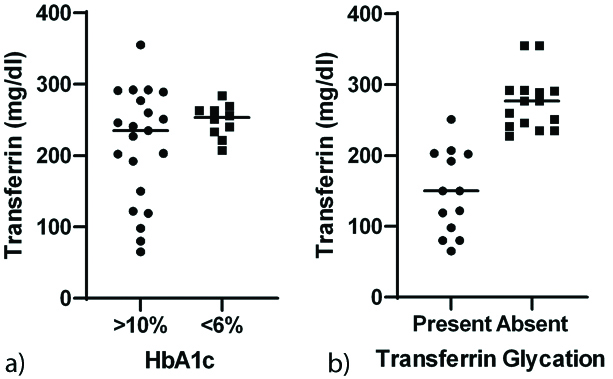
Transferrin glycation was inversely associated with serum transferrin levels (r=-0.72, p=0.02) [Table/Fig-4].
Associations between increased HbA1c, transferrin glycation and transferrin levels. a) Low levels of transferrin in subjects with HbA1c >10% compared to those with HbA1c <6%. b) Transferrin glycation associated with lower transferrin levels.
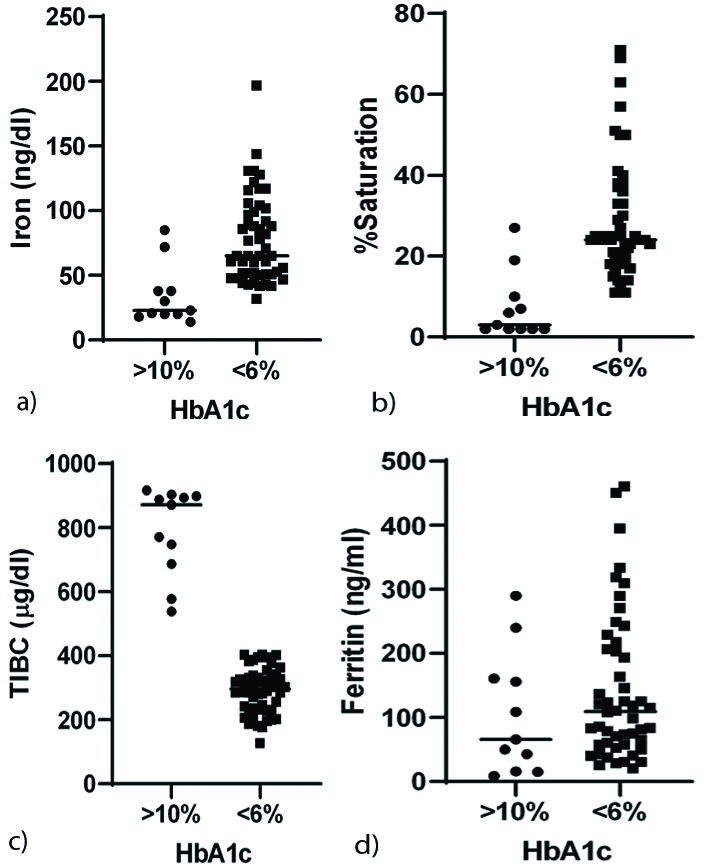
The depletion of transferrin is accompanied by low serum iron, lower percentage saturation and high TIBC in uncontrolled diabetes. No association was observed with ferritin levels [Table/Fig-5].
Perturbations iron metabolism in uncontrolled diabetes. HbA1c >10% showed association with: a) low serum iron; b) lower percentage saturation; c) higher TIBC; and d) null association with Ferritin
Discussion
The current study demonstrates increased incidence of iron deficiency in diabetes subjects with much more increase in uncontrolled diabetes. Iron deficiency was shown to increase HbA1c levels in uncontrolled diabetes. Transferrin saturation percentage decreases with increase in HbA1c. In uncontrolled diabetes, transferrin glycation was associated with depletion of transferrin levels, which in turn deplete iron and percentage saturation; and increase TIBC.
Few studies demonstrated high prevalence of iron deficiencies in diabetic subjects [14]. Non-transferrin bound iron levels were found to be higher in the diabetes, but the total serum iron levels were lower [15]. Insulin resistance was reported to increase with increase in fasting blood glucose, serum ferritin and TIBC [16]. Current study is in agreement with these studies by projecting lower levels of total serum iron and increased TIBC in diabetic subjects. The current study emphasises on frequent monitoring of iron and other biomarkers of iron metabolism specifically in uncontrolled diabetes.
Consistent with the current study, total serum transferrin glycation was shown to increase in diabetes [12]. Glycation of transferrin followed by its increased degradation was observed in type 2 diabetes, which is contributing to systemic oxidative stress [17]. In diabetes, the contribution of transferrin, ceruloplasmin and albumin towards iron-binding antioxidant capacity is lost to a greater extent in comparison to controls [18].
Ferritin was shown to be significantly associated with metabolic syndrome in men and postmenopausal women [19]. In a large scale study on non-diabetic subjects, HbA1c in the highest quintile was shown to be associated with lower levels of haemoglobin, haematocrit and iron compared to HbA1c in the lowest quintile [20]. A high monounsaturated fatty acid supplement specific for diabetic patients was shown to improve HbA1c with specific increase in serum transferrin levels suggesting a possible link of transferrin in glycaemic control [21].
The major strengths of the current study are: i) comparison of markers of iron metabolism across wide range of HbA1c in subjects without any supplementation; ii) demonstration of iron deficiency in elevated HbA1c; and iii) elucidation of transferrin glycation via isoelectric focusing in uncontrolled diabetes. Future studies are warranted to characterise transferrin glycation in uncontrolled diabetes using MALDI-TOF or QTOF analyses.
Limitation(s)
Limited samples were explored for transferrin glycation. Further proteomic analysis to ascertain the glycation sites could not be carried out.
Conclusion(s)
Deficiency of iron and increased TIBC are associated with diabetes with more pronounced effects in uncontrolled diabetes mellitus. Transferin is glycated in uncontrolled diabetes and is associated with significant elevation of TIBC and lower serum transferrin levels. Increased transferrin glycation and decreased serum free transferrin receptors cause iron deficiency anaemia, specifically, in uncontrolled diabetes and hence emphasis should be given on correcting iron levels during management of uncontrolled diabetes.
Simple logistic regression followed by Chi-square test were performed to explore the trend with changing HbA1c levels.
SD: Standard deviation