High Grade Serous Carcinoma Presenting as a Huge Pelvic Mass with Normal Looking Ovaries: A Diagnostic Challenge for Pathologist
Vaishali Walke1, Amrapali Gaikwad2, Madiha Shaikh3, Balwant Kowe4
1 Associate Professor, Department of Pathology, Indira Gandhi Government Medical College, Nagpur, Maharashtra, India.
2 Assistant Professor, Department of Pathology, Indira Gandhi Government Medical College, Nagpur, Maharashtra, India.
3 Resident, Department of Pathology, Indira Gandhi Government Medical College, Nagpur, Maharashtra, India.
4 Professor and Head, Department of Pathology, Indira Gandhi Government Medical College, Nagpur, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Amrapali Gaikwad, 40, Vishwakarma Nagar, Road No. 1, Nagpur-440027, Maharashtra, India.
E-mail: dramrapali.gaikwad@gmail.com
High-grade serous carcinoma represents 50-60% of all ovarian cancers and is the most common type of malignant surface epithelial tumour. Serous carcinoma is often diagnosed in the sixth and seventh decade, while the mean age for high-grade tumours is 63 year. It’s not only the non-specific symptoms like vague abdominal pain, nausea, gastrointestinal disturbances, but also the unusual presentation which is responsible for delayed diagnosis. This diagnostic delay can become the reason for increased mortality, despite advances in surgical management and chemotherapy. Here, authors discuss a case of 60-year-old postmenopausal women who presented with a huge pelvi-peritoneal mass and with grossly normal ovaries. Considering clinical findings and histomorphology, the possibility of malignant mesothelioma was considered as the first differential diagnosis; however extensive sampling of ovaries and supportive immunohistochemical markers helped us to arrive at a definitive diagnosis. The present case emphasises the importance of extensive tissue sampling and ancillary techniques in arriving at a correct diagnosis.
Malignant mesothelioma, Ovarian carcinoma, Peritoneal, Serous carcinoma
Case Report
A 60-year-old woman presented with complaint of gradually progressing lump in abdomen and constipation. There was no significant past history. No history suggestive of diabetes, hypertension or any associated comorbid conditions was obtained. Per abdominal examination, revealed a huge mass filling the pelvis and lower abdomen, extending 1 cm above umbilicus. It was firm, mobile and nontender. There was no evidence of ascites or any organomegaly. Other systemic examination was unremarkable. Intraabdominal lymph-nodes were not palpable. The CT scan of abdomen showed a large 12.2×6.5×3.7 cm sized lobulated pelvico-abdominal mass of heterogenous density with necrotic areas. The ovaries could not be visualised separately from mass. Based on clinico-radiological features, the differential diagnosis considered were malignant mesothelioma, ovarian malignancy.
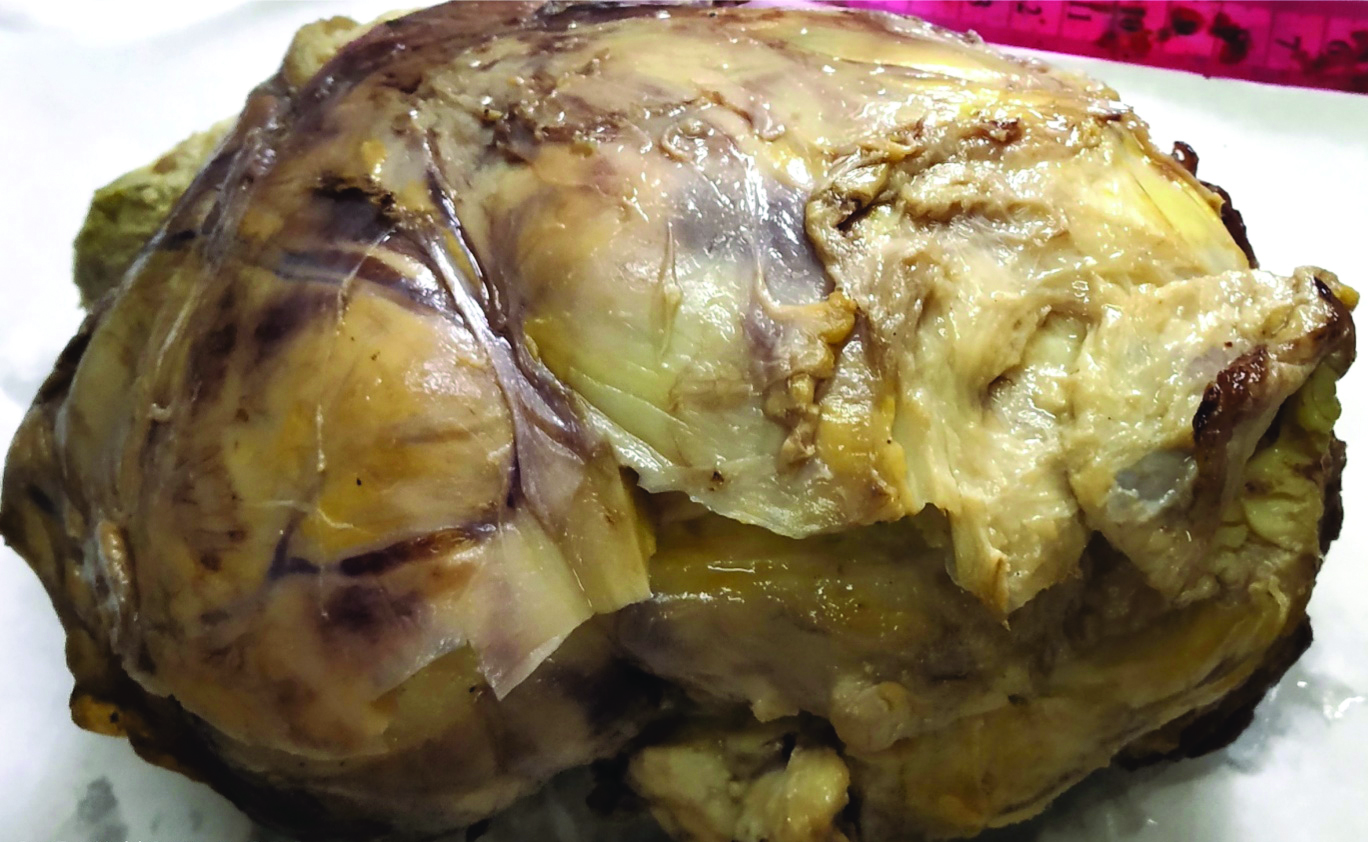
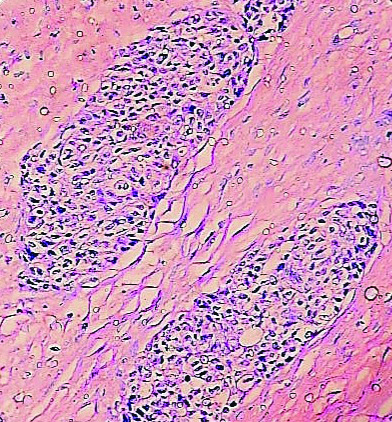
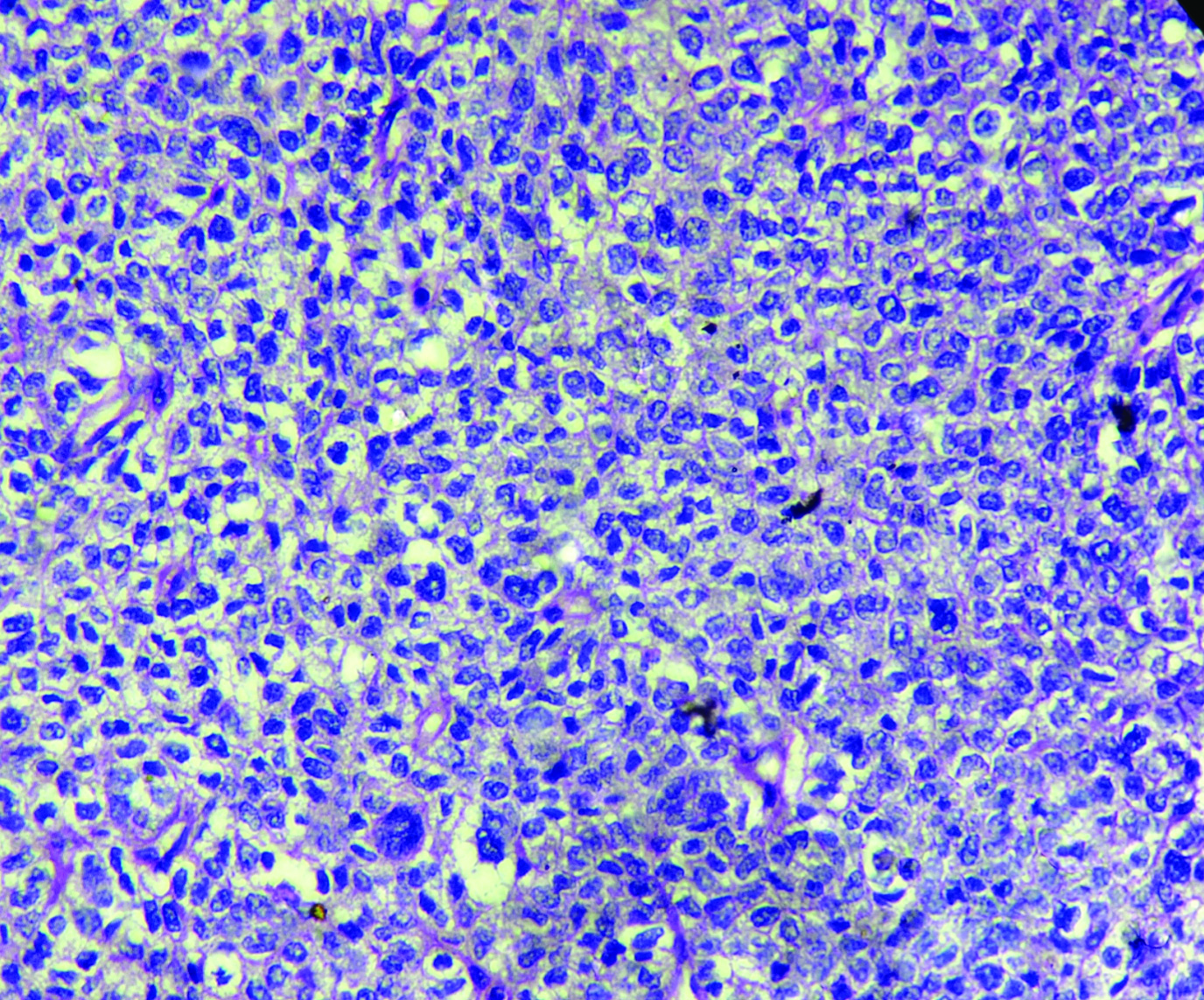
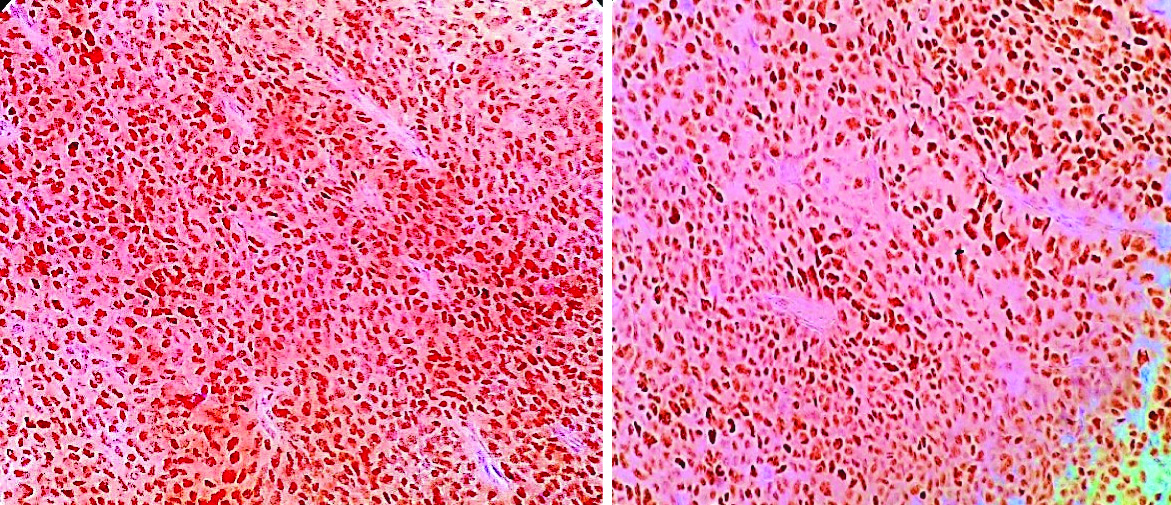
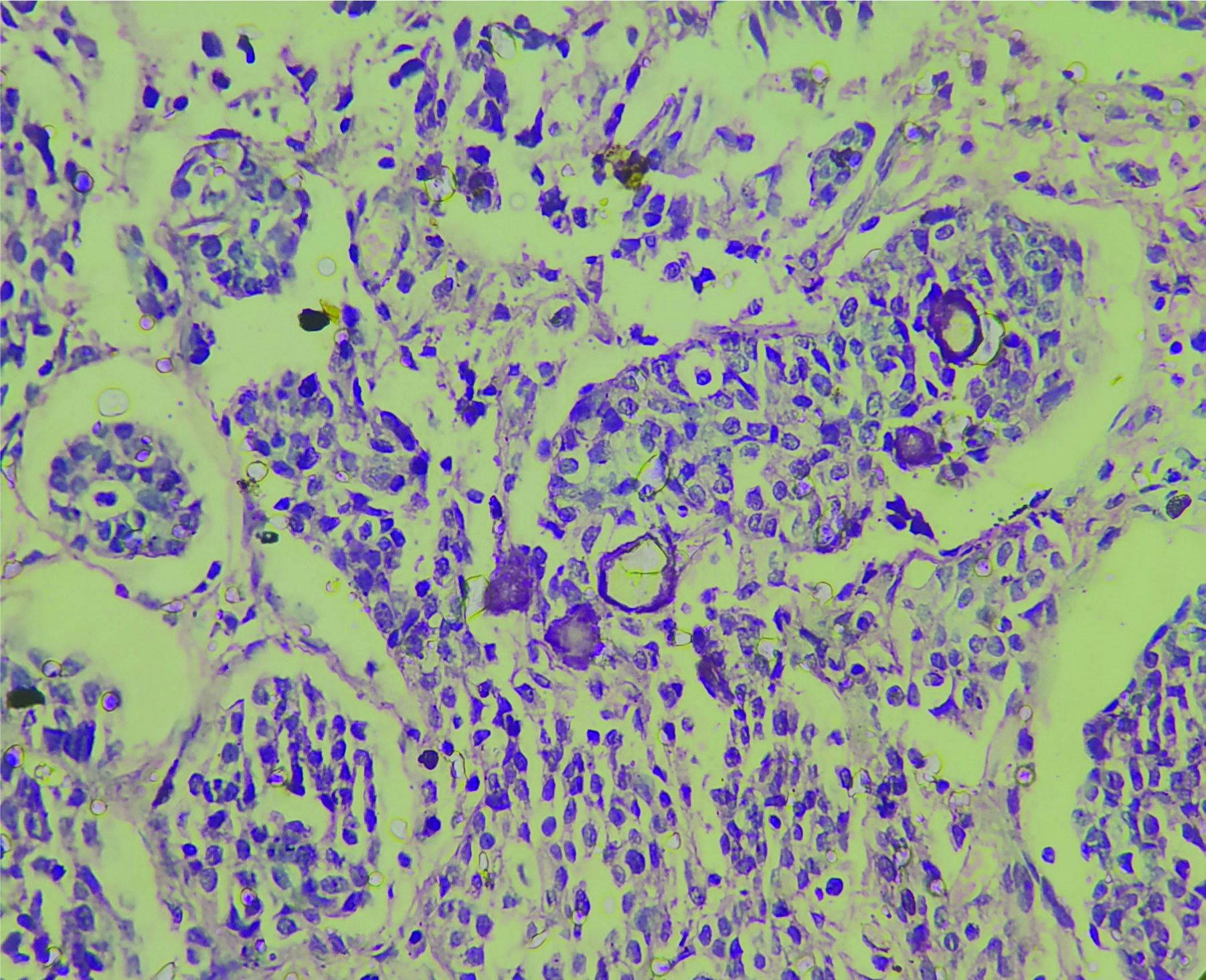
The patient underwent total hysterectomy with bilateral salphingooophorectomy, omentectomy with excision of pelvic mass. Specimen received was a large pelvic mass [Table/Fig-1] measuring 13×7×4 cm, soft to firm with intact capsule. The cut surface was solid, homogenous, greyish white with areas of necrosis. The uterus and both the adnexa were normal on gross examination. The tumour displayed solid sheets and islands, separated by connective septa [Table/Fig-2]. The tumour cells were round with a moderate amount of pale cytoplasm having enlarged pleomorphic nuclei with coarse chromatin and prominent nucleoli. Mitotic figures >5/hpf and necrosis were evident [Table/Fig-3]. The papillary structures could not be identified inspite of extensive tumour sampling. The initial sections from ovaries were unremarkable on histology. The omentum revealed evidence of multiple foci of tumour metastases. In consideration with clinicoradiological features and morphology, differentials considered were malignant mesothelioma and serous carcinoma whether primary or metastatic. The panel of Immunohistochemical markers was applied. The calretinin, D2-40 were immunonegative and therefore ruled out the possibility of malignant mesothelioma; while diffuse nuclear positivity for Wilm’s Tumor Protein 1 (WT1) [Table/Fig-4a] and Paired Box Gene 8 (PAX8) [Table/Fig-4b] favoured the diagnosis serous carcinoma-high grade type. To ascertain whether this serous carcinoma was a primary ovarian carcinoma or a Primary Peritoneal Serous Carcinoma (PPSC) as per the Gynaecology Oncology Group criteria [1], extensive sampling of ovary was done which on serial sections revealed few foci showing papillary fronds with fibro vascular cores covered by multiple layers of highly pleomorphic epithelial cells with vesicular nuclei and one to two nucleoli. Prominent stromal invasion and psammoma bodies were evident [Table/Fig-5]. This tumour was seen to involve the deeper part of ovary more than 5 mm in greatest dimension. These all features supported the final diagnosis of high grade Serous Carcinoma of ovary with metastasis in peritoneum and omentum. After optimal surgical debulking, the patient underwent adjuvant chemotherapy without relapse.
Gross- a large bosselated, pelvic mass.
Tumour cells in nests separated by dense fibrous septa (H&E;10X).
Pleomorphic tumour cells and mitotic figures (H&E; 40X).
a) Nuclear positivity in tumour cells (IHC:WT1;10x); b) Tumour cells showing strong nuclear positivity (IHC:PAX8;10x).
Tumour cells in papillary fronds surrounded by clear spaces and psammoma bodies (H&E; 40x).
Discussion
Ovarian cancer is the most common cause of death in patients with gynaecologic malignancies and requires cytoreductive surgery for adequate treatment [2]. Serous carcinoma of ovary is divided into high-grade and low-grade serous carcinomas [3].
High-Grade Serous Ovarian Carcinomas (HGSOCs) comprise the majority of Serous Ovarian Carcinomas (SOCs), accounting for 80-90% and are most aggressive [4]. HGSOC appears to arise from ovary, fallopian tube or peritoneum [5]. These high grade tumours pose diagnostic difficulty when the clinical presentation is aberrant. The higher mortality associated with HGSOC patients is due to its inability to diagnose the disease in an early stage. The overall prognosis of HGSOC is poor, due to rapid growth rate and lack of specific symptoms. The 10-year survival of patients diagnosed with early-stage HGSOC is 55% and about 15% for those with an advanced-stage disease [6]. Optimal surgical debulking and sensitivity to platinum based chemotherapy is associated with significantly better overall survival. Most ovarian cancer patients initially have high response rates, but later, recur and require further treatment. About 1% cases of ovarian cancer have normal sized ovary [7]. The chances of developing ovarian cancer are more in patients with a significantly enlarged ovary [8], but ovarian cancer in a normal ovary as seen in our case, is rarely suspected.
HGSOC usually spreads by direct extension to adjacent organs in the peritoneal cavity or through detachment of the primary tumour cells. Omental metastases are seen in about 80% of patients with HGSOC. There are currently no effective screening strategies for early detection of ovarian cancer. The CA125 is not specific for epithelial ovarian cancer, as it can be minimally elevated in non malignant conditions like endometriosis and pelvic inflammatory disease, however, it is markedly increased in malignancies including endometrial and pancreatic cancers [9]. Three screening tests are currently used for its early detection such as bimanual pelvic examination, Cancer Antigen (CA) 125, and transvaginal ultrasound [10].
A recent trial evaluating the utility of transvaginal ultrasonography in combination with serum CA125 levels demonstrated some promise in terms of early detection but failed to improve patient outcomes [6].
The correct diagnosis of serous carcinomas is challenging when there is deviation in clinical manifestation. The presentation of a huge pelvic-peritoneal mass made us to consider malignant mesothelioma-epithelial type as the first differential, even though we could not appreciate the papillary structures. Malignant mesotheliomas are the tumours arising from serous surfaces and are aggressive in nature. The incidence of peritoneal mesothelioma is approximately one per 1,000,000 and makes up approximately one-fifth to one-third of all mesotheliomas. When the ovaries appear grossly normal and presentation is of a diffuse peritoneal mass; it closely mimics PPSC; more so as in both the conditions papillary structures are present. In such situations it’s the Immunohistochemistry (IHC) which solves the dilemma [11]. The calretinin and D2-40 immunoreactivity will favour mesothelioma over carcinoma [12].
PPSC is a rare primary malignancy with diffuse involvement of the peritoneum, with minimal or no ovarian involvement, and is clinically indistinguishable from primary SOC [13].
PPSC and HGSOC have common histologic appearance and should be considered in the differential diagnosis. The epithelial layer of ovary and peritoneum are derived from coelomic epithelium early in life. The PPSC involves peritoneal surface extensively, in the absence of an obvious primary site and grossly normal looking ovaries. Hence, also known as normal sized ovarian carcinoma syndrome [14]. The diagnosis of PPSC is based on Gynaecology Oncology Group criteria, which states that: i) The ovaries are either of normal size or enlarged due to benign process; ii) the extraovarian site involvement is greater than surface involvement of either ovary; iii) Microscopically, the ovaries are not involved with the tumour or exhibit only serosal or cortical invasions with dimensions less than 5×5 mm; and iv) The histopathological and cytological characteristics of the tumour are predominantly of the serous type [1].
In any postmenopausal woman presenting with peritoneal thickening, omental nodules, and ascites along with or without ovarian involvement clinically and radiologically, PPSC should be considered in the differential diagnosis. In our case, the ovaries appeared normal, only after extensive sampling and search we could identify the foci of serous carcinoma measuring more than 5 mm in greatest dimension, which established the diagnosis of SOC over that of PPSC.
The staging, as well as treatment of PPSC is similar to HGSOCs (stage III or IV), however some studies suggested worse prognosis in PPSC with median survival between 7 and 27.8 months, while 5 years survival rate from 0% to 26.5% [14].
In the current case, tumour cells showed positivity for PAX8 and WT1, which supported serous carcinoma over mesothelioma. However, PAX8 is expressed in ovarian and endometrial carcinoma- type II [15], whereas WT1 is expressed in ovarian serous tumours and both these markers are useful to support ovarian origin [16]. The present tumour fulfils the criteria laid down by Gynaecology Oncology Group and expression of PAX8 and WT1, all together helped us to arrive at the correct diagnosis.
Conclusion(s)
Primary ovarian carcinoma should be considered in a woman with large peritoneal mass and even with grossly normal appearing ovaries. Authors wish to re-emphasise the role of extensive sampling while dealing with such huge peritoneal masses particularly in female patients in establishing not only an early but an accurate diagnosis which is essential for prompt, appropriate treatment that can further improve the patient’s prognosis.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 06, 2020
Manual Googling: Jun 20, 2020
iThenticate Software: Jul 24, 2020 (18%)
[1]. Irving JA, Young RH, Clement PB, PeritoneumIn: Sternberg’s Diagnostic Surgical Pathology 2015 6th edPhiladelphiaWolters Kluwer:2697-98. [Google Scholar]
[2]. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012Eur J Cancer 2013 49(6):1374-403.10.1016/j.ejca.2012.12.02723485231 [Google Scholar] [CrossRef] [PubMed]
[3]. Kurman RJ, Shih IM, The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theoryAm J Surg Pathol 2010 34:433-43.10.1097/PAS.0b013e3181cf3d7920154587 [Google Scholar] [CrossRef] [PubMed]
[4]. Kurman RJ, Shih IM, The dualistic model of ovarian carcinogenesis. Revisit, revised, and expandedAm J Pathol 2016 186:733-47.10.1016/j.ajpath.2015.11.01127012190 [Google Scholar] [CrossRef] [PubMed]
[5]. Kim J, Park EY, Kim O, Schilder JM, Coffey DM, Cho CH, Cell origins of high-grade serous ovarian cancerCancers (Basel) 2018 10(11):43310.3390/cancers1011043330424539 [Google Scholar] [CrossRef] [PubMed]
[6]. Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C, High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpointsInt J Mol Sci 2019 20(4):95210.3390/ijms2004095230813239 [Google Scholar] [CrossRef] [PubMed]
[7]. Feuer GA, Shevchuk M, Calanog A, Normal-sized ovary carcinoma syndromeObstet Gynaecol 1989 73:786-92. [Google Scholar]
[8]. American College of Obstetricians and Gynaecologists’ Committee on Practice Bulletins-GynaecologyGynaecologists’ Committee on Practice, Practice Bulletin No. 174: evaluation and management of adnexal massesObstet Gynaecol 2016 128:210-26.10.1097/AOG.000000000000176827776072 [Google Scholar] [CrossRef] [PubMed]
[9]. Bast RC Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancerN Engl J Med 1983 309(15):883-87.10.1056/NEJM1983101330915036310399 [Google Scholar] [CrossRef] [PubMed]
[10]. Jelovac D, Armstrong DK, Recent progress in the diagnosis and treatment of ovarian cancerCA Cancer J Clin 2011 61(3):183-203.10.3322/caac.2011321521830 [Google Scholar] [CrossRef] [PubMed]
[11]. Kapoor R, Kuttikat PG, Vaiphei K, Rai B, Patel FD, A case report of peritoneal malignant mesothelioma presenting as primary ovarian massJ Can Res Ther 2015 11:65410.4103/0973-1482.13938226458642 [Google Scholar] [CrossRef] [PubMed]
[12]. Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ, Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesotheliomaMod Pathol 2005 18(1):105-10.10.1038/modpathol.380025915389250 [Google Scholar] [CrossRef] [PubMed]
[13]. Bhanvadia VM, Parmar JK, Madan YG, Sheikh SS, Primary peritoneal serous carcinoma: A rare case and palliative approachIndian J Palliat Care 2014 20:157-59.10.4103/0973-1075.13265325125875 [Google Scholar] [CrossRef] [PubMed]
[14]. Heda K, Indushekar V, Pachori G, Sharma A, Primary peritoneal serous carcinoma: A diagnostic dilemma of pelvic epithelial neoplasmsClin Cancer Investig J 2015 4:551-54.10.4103/2278-0513.157944 [Google Scholar] [CrossRef]
[15]. Yemelyanova A, Gown AM, Wu LSF, Holmes BJ, Ronnett BM, Vang R, PAX8 expression in uterine adenocarcinomas and mesonephric proliferationsInt J Gynaecol Pathol 2014 33(5):492-99.10.1097/PGP.0b013e3182a54afa25083965 [Google Scholar] [CrossRef] [PubMed]
[16]. Kriplani D, Patel MM, Immunohistochemistry: A diagnostic aid in differentiating primary epithelial ovarian tumours and tumours metastatic to the ovarySouth Asian J Cancer 2013 2(4):254-58.10.4103/2278-330x.11988824455652 [Google Scholar] [CrossRef] [PubMed]