HSK is most commonly diagnosed as an incidental finding when patients undergo imaging studies of the abdomen for other reasons as most patients are asymptomatic otherwise [1]. Frequently, the kidneys are united at their inferior poles by an isthmus, which could be functional or non-functional. Rarely, the union occurs at the superior poles, resulting in an inverted HSK. The incidence of this anomaly is 1 in 400 to 600 with a predilection for men [2,3].
Multi-slice CT with high spatial and temporal resolution has become the investigation of choice for evaluation of urinary tract anatomy and pathology especially in HSK [4]. Systematic spiral CT detection of variations of the vascular anatomy is crucial to avoid injuries during various surgical procedures like nephrectomy, renal transplantation and other endovascular procedures.
The HSK is formed by fusion across the midline of two distinct functioning kidneys with one on either side of midline. They are connected by an isthmus of either functioning renal parenchyma or fibrous tissue [5]. In the vast majority of the cases, the fusion is between the lower poles (90%) [4,6]. In the remaining 10%, the superior or both the superior and inferior poles are fused.
Normally, the kidneys ascend and get placed inferior to the adrenal glands on either side. This normal ascent is however restricted in the case of HSK, as the Inferior Mesenteric Artery (IMA) becomes a barrier for the isthmus, leading to low lying kidneys with the lower poles pointing medially [6]. This forces the ureters to course anterior to the isthmus. This anomalous ascent with limited rostral migration predisposes to a primitive arrangement, which also results in renal vascular anomalies, in the form of multiple renal arteries arising from the distal aorta or iliac arteries [7,8].
Materials and Methods
This was a retrospective study done in Sri Muthukumaran Medical College Hospital & Research Institute, Chikkarayapuram, Chennai, from March 2019 to October 2019. CT KUB scans of 861 patients (508 males and 353 females) who were referred to the Radiology Department from the urology clinic from January 2017 to December 2018 were studied. All patients who were referred to the Department of Radiology for CT KUB examinations were included in the study. Since all the patients had satisfactory CT KUB study, no patient was excluded from the study. Being a retrospective observational study, a waiver was granted by the Ethical Committee.
Computed Tomography Examination Protocol
The study included a review of both plain and contrast enhanced CT KUB examinations which were done in patients presenting with urological symptoms. The Radiology Department in the institute routinely obtains written informed consent from the patients prior to contrast study and ensures presence of emergency kit. Axial raw data base was acquired with 64 slice Siemens Somatom Definition-AS with the slice thickness of 5×5 mm and reconstruction interval of 5×5 mm. All contrast enhanced studies were done by injecting intravenous non-ionic iodinated contrast (Iohexol with iodine concentration of 350 mg/mL, at a dose of 1.5-2 mL/kg body weight and injected at a speed of 3-4 mL/second with a power injector (Imaxeon). The CT images were obtained in a cranio-caudal direction in arterial, cortico-medullary and urographic phases with a delay of 10, 30 and 180 seconds respectively. The images were analysed using multiplanar imaging techniques.
Image Interpretation
The raw axial CT images of HSK patients were processed to obtain 3D reconstruction with Maximum Intensity Projection (MIP) and Volume Rendering Technique (VRT). Images were interpreted and analysed by two radiologists with 5-10 years of experience. The anatomical variations were analysed using criteria defined by Kawada S et al., the classification of renal arterial anatomy described by Graves FT and the classification described by Matsumoto T et al., [10-12].
The narrowest part of the connection between the halves of the kidney was considered the renal isthmus [Table/Fig-1]. For deciding the fusion site (the isthmus), we measured a maximum diameter of the outline of the HSK on the axial image and decided the center of the kidneys. If the position of the minimum width of the renal isthmus was less than 5 mm on both sides from the center, it was considered as midline fusion [10].
Contrast enhanced axial CE KUB at the level of the maximum transverse diameter of kidneys, (in one of the patients in this study) shows the narrowest part of the connection between the two halves of the HSK, the renal isthmus. The arrow head shows the thick isthmus.
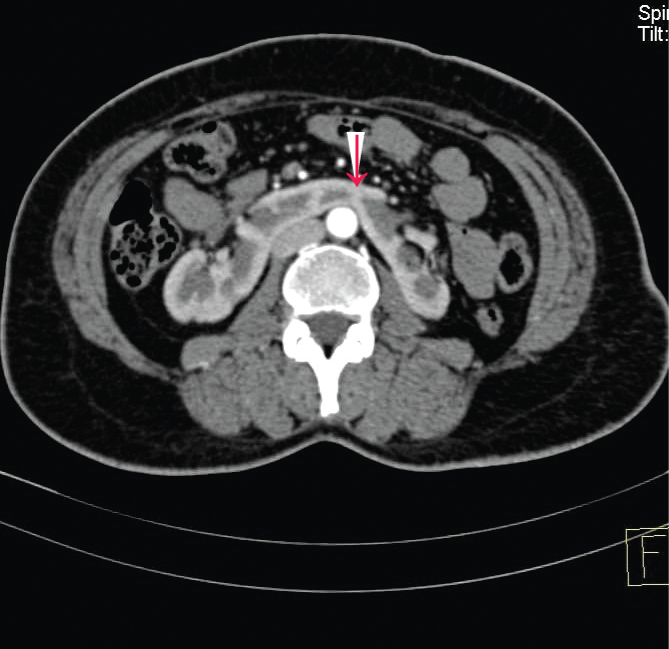
If the narrowest part of the isthmus was more than 5 mm from the midline on the right or left side, this was defined as right and left lateral fusion, respectively.
The HSK was classified based on fusion site and type of isthmic tissue connecting two kidneys [12].
Statistical Analysis
The collected data was entered in MS Excel, Windows 10 and the data was analysed in terms of numbers and percentages.
Results
Four of the 861 patients who had the CT examinations of KUB area had HSK (0.46%). The male to female ratio was 3:1 (males-75%, females-25%). Mean age at the time of diagnosis was 39 years with the standard deviation of 9.96.
In all these four patients, the inferior poles of the kidneys were connected by an isthmus and were classified as Type A(b) according to the classification developed by Matsumoto T et al., [Table/Fig-2] [12].
Comparison of findings in the current study with other study in Indian population. (Based on the classification of Matsumoto T et al., [12]).
| Type | Fusion site/features of isthmus | Findings of Sharma V et al., [9] | Number of patients current study |
|---|
| A(a) | Superior poles | 0 | 0 |
| A(b) | Inferior poles | 7/7 | 4/4 |
| B(a) | Fibrous tissue | - | 0 |
| B(b) | Parenchymal | 7/7 | 3/4 |
| B(c) | Mediators | - | 0 |
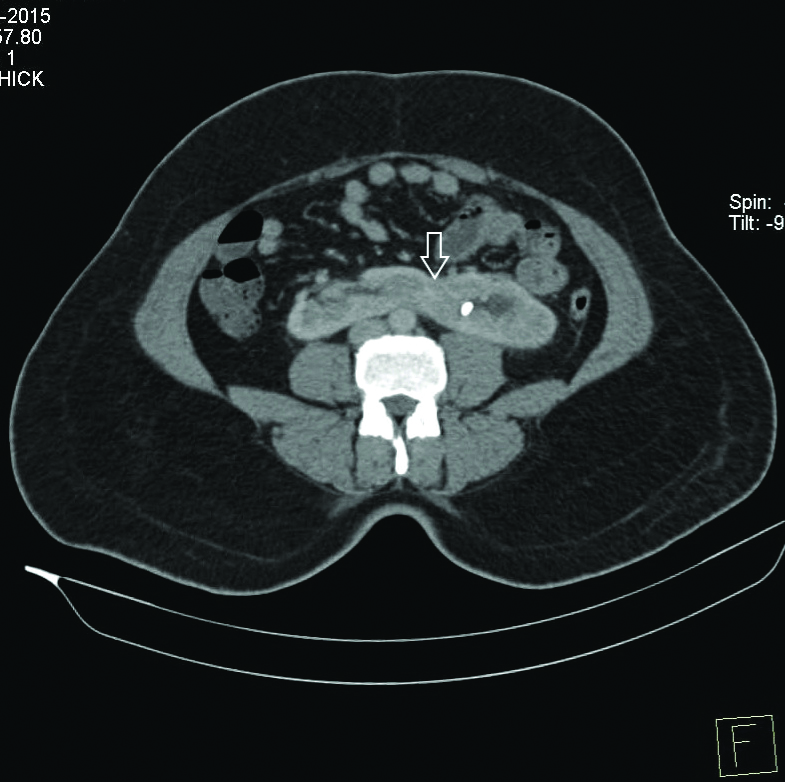
The isthmus was in the midline in one patient ([Table/Fig-1], symmetrical U shaped kidney) and left lateral in 3 patients [Table/Fig-3], asymmetrical L-shaped horseshoe kidney) [Table/Fig-3]
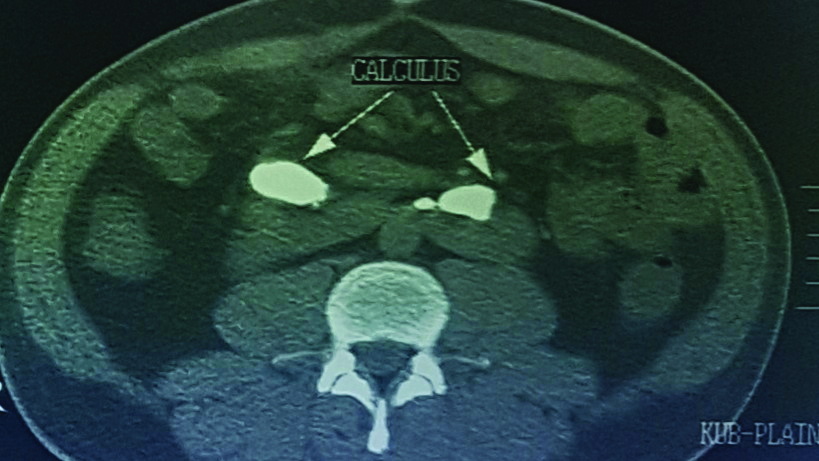
Contrast enhanced CT KUB (CECT) axial section with the arrow showing left lateral fusion with thick isthmus and a left renal calculus.
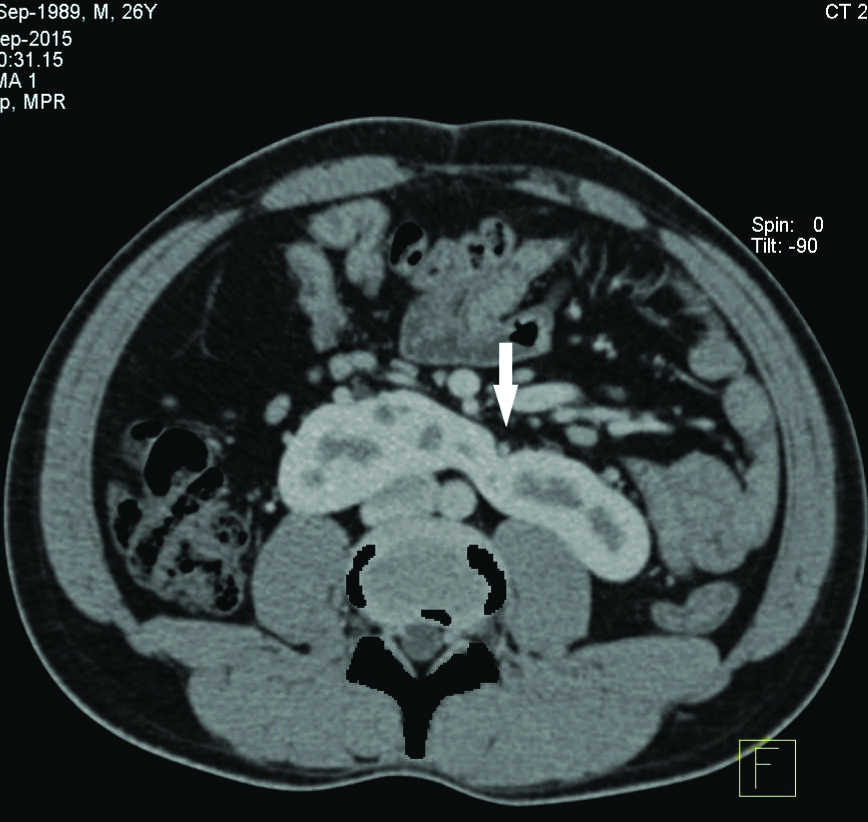
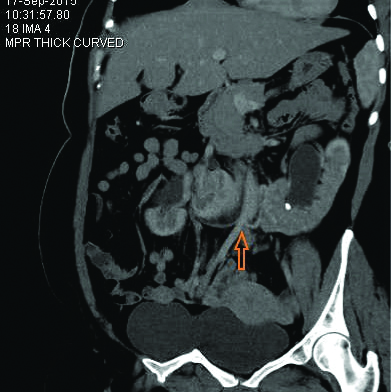
Since one of the four patients in the present study did not undergo a contrast enhanced study, functional status of the isthmus was assessed in the rest of the three patients. All the three patients who underwent contrast CT KUB had a functioning isthmus [Table/Fig-4], Type B(b) as per Matsumoto T et al., [12]. The isthmus was found at L4 level in three patients and L3 level in one patient.
CECT axial section showing functioning isthmus (arrow head).
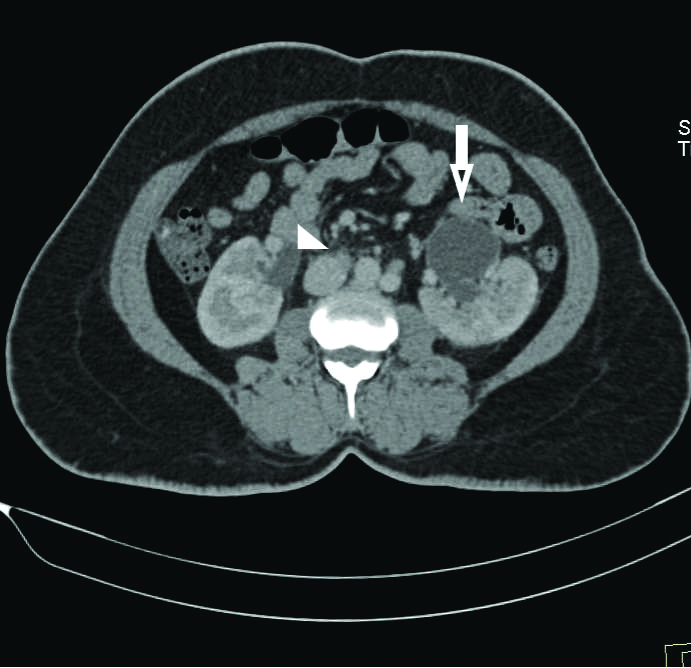
Among the four patients, two had thick isthmus (11±5.6 mm) with associated anteriorly facing renal pelvis. These patients also had mild to moderate PUJO and urolithiasis [Table/Fig-5,6 and 7] at the time of presentation. The other two patients had a thin isthmus (9.5±5.1 mm) and did not have urolithiasis or PUJO.
Incidence of various anatomical variants and complications seen in the current study.
| Characteristics | Number of patients |
|---|
| Midline fusion | 1/4 |
| Left lateral fusion | 3/4 |
| Right lateral fusion | 0 |
| Thin isthmus | 2 |
| Thick isthmus | 2 |
| Renal calculus | 2/4 |
| PUJO | 2/4 |
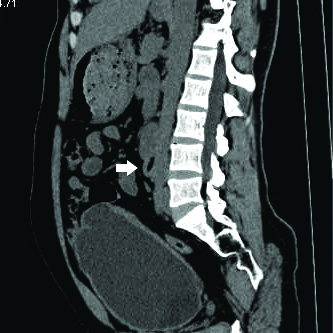
CECT axial section showing bilateral anterior renal pelvis with associated PUJO (left (arrow) >right (arrow head).
Non-contrast CT showing bilateral PUJO with staghorn calculi (white arrows) in anteriorly facing renal pelvis.
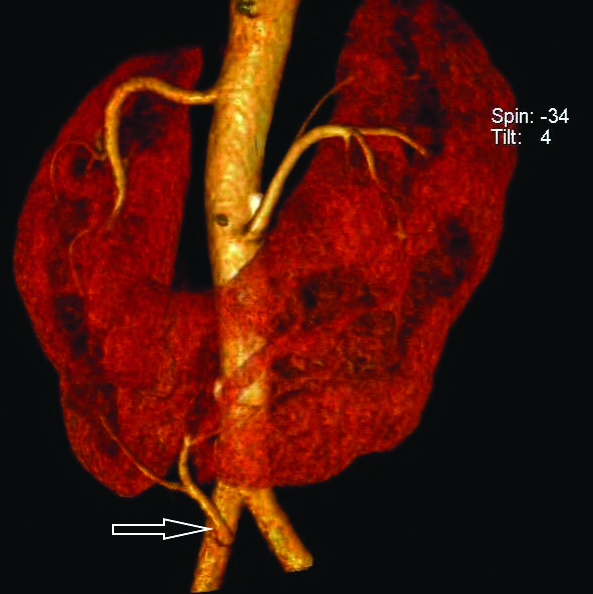
All the four patients in the present study showed variant vascular anatomy in the form of additional caudad isthmic branch arising from the ventral aorta or other major vessels.
One of the four patients showed an isthmic branch arising as a common trunk from the ventral aorta (Graves type 1e, [Table/Fig-8]) with the other three having isthmic branches from the right or left common iliac artery (Graves type 1f). Of the three patients, two had isthmic branches from right common iliac artery either as a common trunk [Table/Fig-9a] or as independent branches [Table/Fig-9b] and one had a common trunk arising from left common iliac artery [Table/Fig-9c]. Accessory left renal artery was present in one patient [Table/Fig-10]. One patient had a retro-aortic left renal vein [Table/Fig-11].
Non-contrast sagittal reconstructed image showing an isthmic branch (white arrow) form the ventral aspect aorta arising just above its bifurcation.
Coronal (Volume Rendering Technique) image showing isthmic branch arising as a common trunk from right common iliac artery (arrow).
Reconstructed volume rendered coronal section showing independent isthmic branches from right common iliac artery (arrow).
CECT reconstructed coronal Maximum Intensity Projection (MIP) showing isthmic branch from left common iliac artery (arrow).
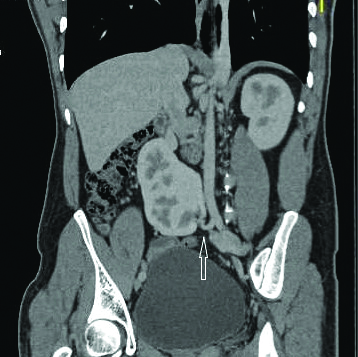
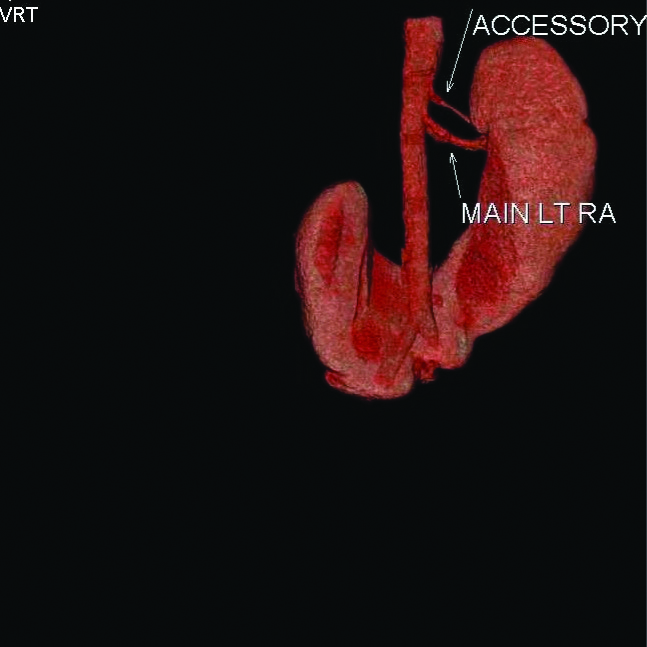
Volume Rendering Technique (VRT) showing accessory left renal artery.
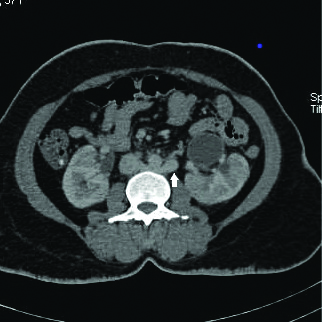
Axial contrast enhanced section of abdomen at L2 vertebral level showing retroaortic left renal vein (arrow).
Discussion
HSK is the most common congenital renal fusion anomaly, accounting for 90% of all fusion anomalies [1]. The prevalence varies from 1 in 400-600 in general population with a male to female ratio of 2:1 [1]. A prevalence of 1 in 304 was noted in patients reporting to urologic clinics [6].
Different methodologies have been used to assess the prevalence of HSK [4,9,13-16]. The [Table/Fig-12] shows the variation in prevalence of HSK in these studies and also compares them with that of the current study.
Comparison of prevalence of HSK between the current study and other studies.
| Study | Methodology | Prevalence of HSK |
|---|
| Sharma V et al., [9] | Multidetector Computed Tomography | 1/97 (1.03%) |
| Williams NS [13] | Necropsy | 1/1000 (0.1%) |
| Dees JE [15] | Pyelography | 1/300 (0.33%) |
| Asghar M and Wazir F [16] | Ultrasonography/Contrast urography | 1/3000 (0.033%) |
| Weizer AZ et al., [14] | Radiographic data analysis | 1/666 (0.15%) |
| Glodny B et al., [4] | Sonography | 1/708 (0.14%) |
| Glodny B et al., [4] | Computed tomography | 1/474 (0.21%) |
| Current study | Multidetector Computed Tomography | 1/215 (0.46%) |
In the present study, we detected HSK kidneys in 4 out of 861 patients (0.46%; 3 males and 1 female) with an estimated prevalence of 1 in 215 patients. The prevalence is higher in this study compared to other studies (excepting one) probably because the study population was limited to patients presenting with urological complaints. The mean age at the time of diagnosis was 39 years which correlated with other studies done in adult populations [4,17].
The renal arterial anatomy was assessed following the basic pattern described by Graves FT [11] [Table/Fig-13].
Comparison of variant vascular anatomy of HSK. (According to Graves classification of renal arterial supply [11]).
| Type | Definition of arterial supply | Findings of the previous literature [22] | Findings of this study n(%) |
|---|
| 1a | Single RA to (U), M & L segments. | 18% | - |
| 1b | Single RA to U & M segments.Separate branch from AA* supplies each L segment. | 6% | - |
| 1c | Single RA to U & M segments. Artery to L segments arise from AA as a common trunk. | 3% | - |
| 1b-1c | Overlapping features of 1b and 1c | 17% | - |
| 1d | U, M & L segments supplied by separate arteries from AA | 4% | |
| 1e | Additional isthmic branches arising from AA below the isthmus. | 28% | 25% (1) |
| 1f | Additional isthmic branches arising from CIA*, IIA*, median sacral artery* | 24% | 75% (3) |
*AA: Abdominal aorta; CIA: Common iliac artery; IIA: Internal iliac artery; Number of patients is indicated in parentheses; U: Upper; RA: Renal artery; M: Middle; L: Lower
The most common fusion site was at the lower poles, in more than 90% of the cases with kidneys appearing more vertical than normal due to medial orientation of lower poles [4,9,10]. In the present study, all the four patients showed fusion at the lower poles. This is in agreement with Sharma V et al., [9] who in their retrospective study on 682 patients, found all the 7 patients with HSK to have fusion at lower poles (Type A(b)) [Table/Fig-2]. Previous studies have shown that isthmus was made of functioning parenchyma in the majority of the patients with approximately 15% of them showing non-functioning isthmus [4,9,10]. Similarly in the present study, all the three patients who underwent contrast enhanced study showed a functional isthmus (Type B(b) as per classification of Matusmoto T et al.) [12].
Left lateral fusion (asymmetrical L shaped kidney) occurs in 50-70% of the HSK which is attributed to the location of the isthmus anterior to the aorta which always lies to the left of the midline [4,10]. This study also found left lateral fusion [Table/Fig-3] in 3 out of 4 patients (75%) with midline fusion (symmetrical U shaped) [Table/Fig-1] found in 1 case. No right lateral fusion was found. No signification correlation was found between the fusion site and the complications.
While ascending from pelvis to mid abdomen, the HSK may be found at any level along this path with the isthmus usually lying anterior to the great vessels, at the level of the third to fifth lumbar vertebra [8]. The more superficial and caudad location of the HSK along with absence of rib protection makes it more vulnerable to trauma [18]. The isthmus was found at L4 level in three patients and L3 level in one patient in the present study with all the four patients having low lying kidneys. The halted ascent along with the failure of normal rotation results in change in the longitudinal axis of the HSK with the renal pelvis located anteriorly and the calyces directed medially and inferiorly. Since the pelviureteric junction is at a higher level, the associated high insertion of the ureter and impeded drainage makes it more susceptible for PUJO [2,11].
Among the four patients, two patients who showed thick isthmus(13-15mm) also had anteriorly placed renal pelvis and high insertion of ureter with associated PUJO and urolithiasis [Table/Fig-6,7], whereas those with a thin isthmus (9-10mm) did not have urolithiasis. These findings correlated with those of Kawada S et al., who observed, that malrotation with anterior displacement of the collecting system and superior ureteric insertion into the renal pelvis was severe in cases of HSK with thick isthmus and that thick isthmus was an independent risk factor for urolithiasis [10]. Urolithiasis is quite common in patients with HSK (20-60% of cases) [19]. In the present study, 2 of the 4 patients had urolithiasis with bilateral staghorn calculi seen in one of the patients.
Significant variation in the arterial supply of HSK is reported in majority (70-80%) of the patients [20]. Aberrant renal vasculature poses significant challenges to the operating surgeon making urological procedures, renal transplantation and endovascular procedures on the aorta difficult with injury or ligation resulting in renal infarction [4,20]. In approximately, one-third of the patients with HSK, a single renal artery was found while in the remaining patients, multiple hilar and isthmic vessels arising from the abdominal aorta, iliac arteries, or IMA were seen [21].
Describing the variations in the arterial supply to HSK, Graves FT recognised six patterns of vasculature [11]. [Table/Fig-13] shows the comparison of the variant vascular anatomy between the present study and the findings of review done by Natsis K et al., [22].
Three out of 4 patients in present study, had isthmic branches arising from the common iliac arteries {type1 f (75%)} and one patient had a ventral branch from the aorta just proximal to its bifurcation {(type 1e,(25%)}. This is similar to the findings of Glodny B et al., who found that variants of the most cephalad arteries on either side were rare [4] with majority of the patients showing type 1f and type 1e [4,11,22]. The study population was also unique in that none of the patients had multiple renal arteries (i.e., >3). This finding was different from previous studies which showed a preponderance of multiple renal arteries [11,22]. One patient had accessory left renal artery ([Table/Fig-10], 30%) which was not higher than the general population.
The incidence of renal (22.9%) and systemic venous anomalies (28.6%) were similar to that in the general population [23]. In the current study, no significant renal venous anomalies were noted except for one patient, who had an anomalous retroaortic left renal vein [Table/Fig-11].
Increased incidence of renal malignancies like renal cell carcinoma, Wilms tumour and carcinoid tumour in horseshoe kidney may be due to chronic obstruction, lithiasis, infections and possibly due to abnormal migration of posterior nephrogenic cells as explained by teratogenic theory [19]. No renal tumour was found in this study. Syndromic association of horseshoe kidneys in one-third of the cases explains its association with other genitourinary, cardiovascular and central nervous system abnormalities [1,2]. No congenital anomalies were found in the present study. This is in agreement with Glodny B et al., who found concomitant malformations are considerably seen more often in paediatric population and are less frequent in adults [4].
Limitation(s)
Only routine contrast enhanced studies were done, we could not acquire renal CT angiographic images. One of the four patients who was diagnosed with HSK did not undergo a contrast enhanced CT study and this study did not include the general population, hence its results cannot be generalised.
Conclusion(s)
Horseshoe Shaped Kidney is the most common congenital fusion anomaly and is more common in males. Though it may remain asymptomatic throughout life and is detected incidentally on imaging, it is associated with wide range of anatomical and congenital anomalies. This study demonstrated that patients with a thick isthmus and associated with anteriorly facing renal pelvis were more prone to develop PUJO and renal calculi. An increased awareness of prevalence of HSK and its anatomical variations will make surgical and endovascular procedures related to the kidney and the major blood vessels, much safer and more precise. MDCT is an invaluable tool in demonstrating these subtle changes in anatomy and blood supply of HSK and thus plays a major role in reducing iatrogenic injuries. It is also an excellent tool in the diagnosis and follow-up of complications and treatable causes of renal pathology in these patients.
*AA: Abdominal aorta; CIA: Common iliac artery; IIA: Internal iliac artery; Number of patients is indicated in parentheses; U: Upper; RA: Renal artery; M: Middle; L: Lower