The CKD is a condition characterised by a gradual loss of renal function over the time which has emerged as a major public health concern affecting approximately 11-13% of the world’s population [1,2]. In Indian population, its prevalence has been reported as 17.2% [3,4]. The Kidney Disease Outcomes Quality Initiative (KDOQI) defines CKD as either kidney damage or a decreased effective Glomerular Filtration Rate (eGFR) of less than 60 mL/min/1.73 m2 body surface areas, for at least three months [1]. KDOQI also gave the guidelines to divide CKD into different stages based on the eGFR levels (as shown below) [Table/Fig-1] [1].
Decrease in renal mass and function in CKD leads to progressive deterioration in mineral homeostasis as shown in the [Table/Fig-2] flowchart below [5,6].
With the progression of CKD, iPTH level goes on rising but is unable to maintain normal levels of Ca, P and ALP [5]. Finally, deranged normal serum and tissue concentrations of all these parameters occur, resulting in a decrease in bone density and calcium deposition in soft tissues. These disturbances have traditionally been termed renal osteodystrophy [7,8].
The term renal osteodystrophy describes only the bony changes of CKD, but not the other pathological changes due to calcium deposition in soft tissues [8]. So, Kidney Disease Improving Global Outcomes (KDIGO) described MBD to include changes made in body due to CKD [9].
MBD is a systemic disorder of mineral and bone metabolism due to CKD. It usually exhibits as an abnormality in the metabolism of ALP, Ca, P, iPTH or 25(OH)D or could be attributed to a defect in the bone turnover, mineralisation, bone volume or any soft tissue calcification [9].
Earlier CKD-MBD was diagnosed with the help of invasive tests like bone biopsy and whole-body irradiation (DEXA scan) [16]. The disease can be diagnosed very easily and accurately with the help of biochemical markers (Ca, P, ALP and iPTH), and there is no need of unnecessary radiation exposure and invasive bone biopsies [17].
Thus, CKD leads to metabolic derangements of mineral and bone homeostasis which in turn significantly affects the quality of life in such patients [10]. This study was designed to calculate the prevalence of MBD in CKD patients by estimation of biochemical markers Ca, P, ALP and iPTH and to analyse the disease burden across different stages of CKD.
Materials and Methods
A hospital based cross-sectional study was conducted at Medanta-The Medicity hospital in 2300 CKD cases who visited Nephrology OPD for follow-up visits from October 2017 to December 2018. As per the medical records obtained from the hospital database, previously diagnosed CKD cases belonging to stages 3, 4, 5 and 5D based on KDIGO criteria i.e., kidney damage or a decreased GFR of less than 60 mL/min/1.73 m2 for at least three months [9] and who were taking all the supplements and medications (oral calcimimetics, Phosphate binders and Vitamin D analogues) as per treatment protocol were included in our study. Patients who had primary hypo or hyperparathyroidism (being managed for the same even before the diagnosis of CKD) or had prior parathyroid surgery, who were having Chronic Liver disease, pregnant women, patients who were having history of fracture within last six months and who were having Rheumatologic diseases were excluded from the study. Ethical approval was obtained from the Institutional Ethics Committee (MICR-803/2017) and written informed consent was obtained from all the participants prior to inclusion in the study.
Biochemical Analysis
After overnight fasting, 5 mL of blood samples were taken, centrifuged to separate the serum and processed for estimating serum Ca, P, ALP and iPTH on a daily basis. Serum Ca, P and ALP were estimated in VITROS 4600 Chemistry Automated Analyser using Arsenazo dye method [18], Phosphomolybdate reduction (PMA Phenol) method [19] and p-nitrophenyl method [20] respectively. iPTH was estimated using Chemiluminescent Microparticle Immunoassay (CMIA) method [21] in ARCHITECT I system autoanalysers.
As per the KDOQI guidelines [1], normal reference ranges used for the purpose of this study were: Ca- 8.4-9.5 mg/dL, P- 2.7-4.6 mg/dL (for stage 3 and 4) and 3.5-5.5 mg/dL (for stages 5 and 5D), ALP- 38-120 U/mL and iPTH- 15-68.3 pg/mL.
The analysis included profiling of patients on different demographic and gender basis. Patients of stage 5D were considered as dialysis-dependent. For each of the stages, prevalence of deranged serum levels of above biochemical markers and MBD was estimated.
Statistical Analysis
Statistical analysis was done using SPSS software, version 24.0. Descriptive analysis of quantitative parameters was expressed as mean and standard deviation. The analysis for comparison among three or more categories was done using one-way ANOVA. For comparison of proportions, cross tables were generated and chi-square test was used for testing of associations. Categorical data was expressed as absolute number and percentage in a contingency table along with the chi-square and p-values. The p-value <0.05 was considered statistically significant.
Results
The study was conducted in 2300 CKD cases with mean age of 53.1±15.4 years. Out of 2300 patients, 1754 (76.3%) were males and 546 (23.7%) were females. Stage wise distribution of the patients is shown in the [Table/Fig-3].
Number (n) and percentage (%) distribution of patients in different stages of CKD.
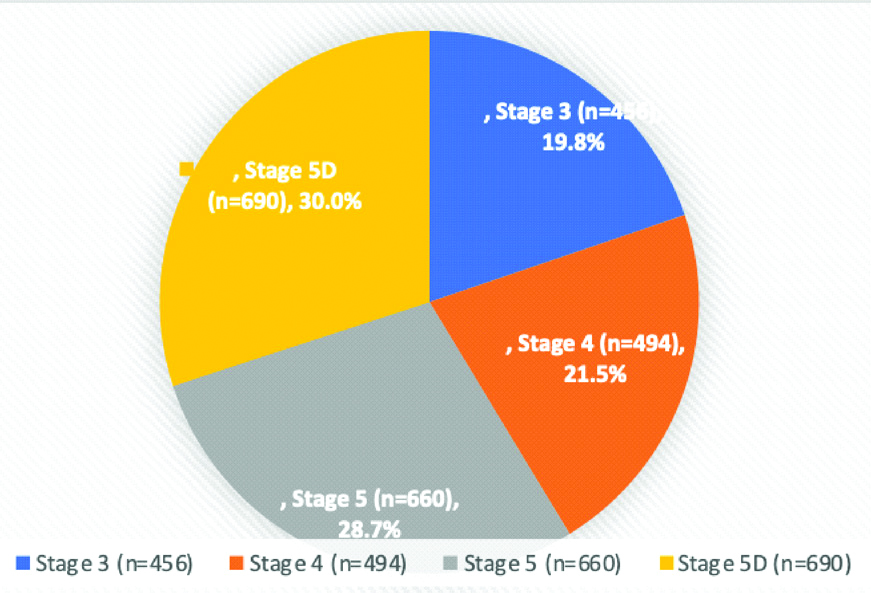
Out of total 2300 CKD cases, 1877 (81.6%) had MBD and 423 (18.4%) were without MBD.
Further, MBD prevalence across different stages of CKD is shown in [Table/Fig-4]. Maximum disease burden was noted in the advanced stages of CKD (stage 5 and 5D). The prevalence of MBD showed an increasing trend across the stages of CKD; the increase being statistically significant as per Chi-Square Test of significance [Table/Fig-4].
Prevalence of MBD across the stages of CKD.
| Stage 3 | Stage 4 | Stage 5 | Stage 5D | Row total |
|---|
| MBD present | 289 (63.4%) | 380 (76.9%) | 578 (87.6%) | 630 (91.3%) | 1877 (81.6%) |
| MBD absent | 167 (36.6%) | 114 (23.3%) | 82 (12.4%) | 60 (8.7%) | 423 (18.4%) |
| Column total | 456 | 494 | 660 | 690 | 2300 |
χ2=172.994; p<0.001**
Chi-square test was used to find out the association between the prevalence of MBD and the stage of CKD which was found to be statistically highly significant (**p<0.001).
[Table/Fig-5] shows the total percentage of patients with biochemical derangements (low Ca, high P, high ALP and high iPTH across the stages of CKD. It was observed that with the progression of CKD stages, the proportion of cases with these biochemical derangements increased; the difference being statistically significant as per the Chi-Square test.
Prevalence of deranged parameters across the various stages of CKD.
| Stage 3 (n=456) | Stage 4 (n=494) | Stage 5 (n=660) | Stage 5D (n=690) | Overall (n=2300) |
|---|
| Calcium (Ca) |
| Hypocalcaemia present | 56 (12.3%) | 92 (18.6%) | 238 (36.1%) | 253 (36.7%) | 639 (27.8%) |
| Hypocalcaemia absent | 400 (87.7%) | 402 (81.4%) | 422 (63.9%) | 437 (63.3%) | 1661 (72.2%) |
| Column total | 456 | 494 | 660 | 690 | 2300 |
| χ2=123.475; p<0.001** |
| Phosphorus (P) |
| Hyperphosphatemia present | 61 (13.4%) | 163 (32.8%) | 439 (66.4%) | 447 (64.8%) | 1110 (48.3%) |
| Hyperphosphatemia absent | 395 (86.6%) | 331 (67.2%) | 221 (33.6%) | 243 (35.2%) | 1190 (51.7%) |
| Column total | 456 | 494 | 660 | 690 | 2300 |
| χ2=434.519; p<0.001** |
| Alkaline Phosphatase (ALP) |
| High ALP present | 76 (16.9%) | 128 (25.9%) | 172 (26.2%) | 234 (33.9%) | 610 (26.5%) |
| High ALP absent | 380 (83.1%) | 366 (74.1%) | 488 (73.8%) | 456 (66.1%) | 1690 (73.5%) |
| Column total | 456 | 494 | 660 | 690 | 2300 |
| χ2=41.295; p<0.001** |
| Intact Parathyroid Hormone (iPTH) |
| Hyperparathyroidism present | 253 (55.5%) | 339 (68.6%) | 553 (83.8%) | 594 (86.1%) | 1739 (75.6%) |
| Hyperparathyroidism absent | 203 (44.5%) | 155 (31.4%) | 107 (16.2%) | 96 (13.9%) | 561 (24.4%) |
| Column total | 456 | 494 | 660 | 690 | 2300 |
| χ2=183.548; p<0.001** |
Chi-square test was used to find out the association between the prevalence of deranged parameters and the stage of CKD; p<0.001** statistically highly significant
There was an increasing trend of biochemical derangements seen in total CKD cases as well as in different stages. Maximum percentage of such cases was seen in stage 5 and 5D for all the above parameters.
The mean values and range of all the four parameters (Ca, P, ALP, iPTH) in our patients is shown in [Table/Fig-6].
Mean values and range of all the parameters (Ca, P, ALP, iPTH).
| Stage 3 (n=456) Mean±SD (Range) | Stage 4 (n=494) Mean±SD (Range) | Stage 5 (n=660) Mean±SD (Range) | Stage 5D (n=690) Mean±SD (Range) | F value; p-value |
|---|
| Ca (mg/dL) | 9.04±0.73 (5.9-11.8) | 8.86±0.89 (5.5-12.8) | 8.58±1.02 (5.4-12.9) | 8.47±0.97 (3.1-12.1) | 51.7; <0.001** |
| P (mg/dL) | 4.02±0.97 (2.4-10.7) | 4.52±1.34 (1.9-12.6) | 5.87±2.05 (2.2-16.2) | 5.92±2.33 (1.9-15.2) | 148.0; <0.001** |
| ALP (U/mL) | 102.24±45.93 (8.1-474.0) | 116.83±67.52 (31.0-793.0) | 117.24±60.10 (32.0-452.0) | 132.96±103.54 (33-1148) | 15.6; <0.001** |
| iPTH (pg/mL) | 145.20±143.93 (5.4-1245) | 212.92±197.47 (7.2-1879) | 403.39±361.69 (9.7-3646) | 430.45±389.49 (6.9-2443) | 90.6; <0.001** |
Intergroup comparison of means of biochemical markers in different stages of CKD was done using One-Way ANOVA; p<0.05* statistically significant; p<0.001**statistically highly significant
Distribution of dialysis dependent and nondialysis dependent patients according to different ranges of iPTH is summarised in [Table/Fig-7]. Out of 2300 patients, 690 were dialysis dependent.
Percentage of dialysis and nondialysis dependent patients with different ranges of iPTH.
| iPTH (pg/mL) | N (No. of patients) | % of patients |
|---|
| Nondialysis dependent (n=1610) |
| <150 | 696 | 43.2% |
| 150-300 | 414 | 25.7% |
| >300 | 332 | 20.6% |
| >600 | 168 | 10.4% |
| Dialysis dependent CKD patients (n=690) |
| 136-614 (2-9 times N upper range) | 344 | 49.9% |
| <136 (<2 times N upper range) | 170 | 24.6% |
| >614 (>9 times N upper range) | 176 | 25.5% |
Discussion
The present study conducted on 2300 CKD cases reported an overall MBD prevalence of 1877 (81.6%) in CKD cases. Similar results were also reported by studies done by Ahmed HAE et al., and Shaheen FA et al., [22,23]. It was seen that despite taking all the necessary medications and supplements as mentioned above, the prevalence of CKD-MBD was very high in almost all the parts of the world [22,23]. A study done in Singapore by Chuang SH et al., reported that a total of 84.9% and 41.9% of the patients were prescribed phosphate binders and vitamin D, respectively and according to the KDOQI targets, the prevalence of CKD-MBD in the study population was 67.4% at baseline, 79.1% at one month and 86.0% at 4-6 months [24]. Similarly, Shaheen FA et al., in their study conducted on 2247 CKD patients of stages 4, 5 and 5D reported an overall MBD prevalence of 83.2% and also reported approximately 85% of their patients had treatment with phosphorous binders [23].
In the present study, it was shown that with the progression of CKD stage, the prevalence of MBD also increased and maximum prevalence of MBD was found in stage 5D which was 91.3%. Our results were in concordance with other studies done by Etta PK et al., and Abrita RR et al., [25,26].
A total of 27.8% of our patients had hypocalcaemia. Similar results were observed in other Indian studies also, wherein hypocalcaemia was observed in 19.2% cases of CKD stage 3-5D by Shankar P et al., and 23.8% in stage 3-5D CKD patients by Vikrant S and Parashar A [27,28]. Similarly, worldwide studies done by Jin JJ et al., Wazir B et al., and Sun YP et al., reported overall hypocalcaemia in 35.9%, 34.3% and 20.3% of their CKD population, respectively [29-31]. Stage wise prevalence of hypocalcaemia in our study population ranged from 12.3% in stage 3 to 36.7% in stage 5D patients (p<0.001**) as shown in [Table/Fig-5]. According to a study done by Shaheen FA et al., KDIGO target for Ca was met by 47.2% of patients with eGFR <30 mL/min/1.73 m2 and 45.4% of dialysis dependent patients [23]. Shankar P et al., reported the prevalence of hypocalcaemia in Stage 3, 4, and 5 as 12%, 11.6%, and 37.5%, respectively, which was very similar to the present study [27].
Melamed ML et al., found that Ca >9.73 mg/dL was associated with 52% increased risk of death compared to reference range (8.97-9.33 mg/dL) [32]. Similarly, it was observed by Floege J et al., that patients with Ca level >10.2 mg/dL and <8.4 mg/dL had a slightly higher risk of death than those who were within target range [33].
There is Phosphorus retention early in the course of CKD [6]. The percentage of patients having hyperphosphatemia in our study was 48.3% as shown in [Table/Fig-5]. Prevalence of hyperphosphatemia reported by various other studies was 74%, 81.8%, 58.6% and 57%, by Ahmed HAE et al., Etta PK et al., Jin JJ et al., and Waziri B et al., respectively [22,25,29, 30]. Shaheen FA et al., reported prevalence of hyperphosphatemia in stage 4, 5 and 5D as 40.6%, 73.4% and 43.7%, respectively, that is comparable to levels reported in the present study [23].
Zhou C et al., concluded that high Phosphorus level is an independent risk factor for abdominal aortic calcification and left ventricular hypertrophy [34]. Similarly, Suki WN and Moore LW, concluded that more than 5.5g/dl Phosphorus levels is associated with an increased risk of cardiovascular and all-cause mortality [35]. Floege J et al., reported a significant increase in Phosphorus levels after a follow-up of 20.9 months in CKD patients and concluded that high level of Phosphorus is a significant risk factor for mortality in CKD patients [33]. So, lowering of serum Phosphorus is the primary strategy to manage advanced stage CKD patients.
Prevalence of high ALP in different stages of CKD in the present study population was: 16.9%, 25.9%, 26.2% and 33.9% in stages 3, 4, 5 and 5D, respectively. Shankar P et al., reported prevalence of high ALP as 8%, 29.4% and 25% in stage 3, 4 and 5, respectively which was similar to the prevalence obtained in our study [27]. It was concluded by Ahmed HAE et al., and Gorriz JL et al., that high levels of PTH is related to high levels of ALP [22,36].
The most common abnormality found in our patients was secondary hyperparathyroidism. According to the cut-off value utilised in the present study, hyperparathyroidism (iPTH >68.3 pg/mL) was present in 75.6% (n=1739) patients. Similarly, hyperparathyroidism (iPTH >68 pg/mL) was reported in 49.5% and 65.06% patients in studies done by Etta PK et al., and Shankar P et al., respectively [25,27]. Waziri B et al., reported hyperparathyroidism (iPTH >150 pg/mL) in 73.4% patients [30].
Taking into account the KDIGO target of iPTH in dialysis dependent patients as 2-9 times normal, 49.9% of our dialysis dependent population was within range. Abrita RR et al., also reported similar results with 49.5% of their dialysis dependent patients having iPTH level within KDIGO range [26].
In the present study, it was observed that, with the progression of CKD, there was a gradual increase in the iPTH level. Gorriz JL et al., and Wang WH et al., also concluded the same, that a decrease in GFR (progression of CKD) is associated with an increased iPTH levels [36,37].
In a study done by Tentori F et al., (DOPPS study) on 35,655 dialysis dependent participants it was observed that iPTH <50 pg/mL and >600 pg/mL contribute to the development of adynamic bone disease, higher risk of hospitalisation, poor quality of health and increased cardiovascular mortality [38]. Floege J et al., also gave similar results, that patients in the highest iPTH category (>600 pg/mL) in their study population experienced a 2-fold increase in risk of death compared to patients who were within target range and the patients in the lowest iPTH category (<75 pg/mL) had almost a 50% greater risk of death than target range patients [33].
In coherence with the global data on prevalence of MBD in CKD available in literature, the results of the present study suggest that a significant population of CKD patients suffer from MBD, which apart from its bone related complications, also makes them vulnerable to cardiovascular complications and its associated mortality. In the wake of this high a prevalence, the need of the hour is to brainstorm a better therapeutic option targeting this vulnerable group.
Limitation(s)
The limitations of the present study are, the biochemical parameters for CKD-MBD were not tested at regular intervals during the study period and a cause-effect relationship could not be established in the study due to single point cross-sectional observational design.
Conclusion(s)
Despite taking all the necessary medications and supplements to prevent MBD, i.e., oral calcimimetics, Phosphate binders and Vit D analogues, the prevalence of CKD-MBD came out to be very high (81.6%). There needs to be some change in the protocol that should be followed universally so as to reduce this huge burden of MBD which adversely hampers the quality of life and attributes to mortality rate in CKD patients.
χ2=172.994; p<0.001**
Chi-square test was used to find out the association between the prevalence of deranged parameters and the stage of CKD; p<0.001** statistically highly significant
Intergroup comparison of means of biochemical markers in different stages of CKD was done using One-Way ANOVA; p<0.05* statistically significant; p<0.001**statistically highly significant