Henoch Schonlein Purpura as Late Manifestation of Hepatitis A Infection
Prateek Jindal1, Kapil Bhalla2, Neha3, Sanjiv Nanda4
1 Junior Resident, Department of Paediatrics, Pt. B. D. Sharma, PGIMS, Rohtak, Haryana, India.
2 Associate Professor, Department of Paediatrics, Pt. B. D. Sharma, PGIMS, Rohtak, Haryana, India.
3 Senior Resident, Department of Paediatrics, Pt. B. D. Sharma, PGIMS, Rohtak, Haryana, India.
4 Senior Professor and Head, Department of Paediatrics, Pt. B. D. Sharma, PGIMS, Rohtak, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Neha, Pt. B. D. Sharma, PGIMS, Rohtak, Haryana, India.
E-mail: nehadoc1991@gmail.com
An uncommon presentation of Hepatitis A Virus (HAV) Infection is cutaneous vasculitis. This report is about a seven-year-old male patient that came to the pediatric emergency with complaints of bilateral swelling of lower limbs and pain in right knee for the last three days. Patient had history of jaundice one month back. On examination, he was febrile and had swelling over right knee with decreased range of movements and multiple palpable purpura over lower extremities and buttocks. Laboratory evaluation showed deranged Liver Function Test (LFT) and positive HAV IgM antibodies. Histopathological examination of skin biopsy was suggestive of leukocytoclastic vasculitis and IgA deposition. Final diagnosis of Henoch Schonlein Purpura (HSP) was established based on clinical findings and skin biopsy findings which are usually sufficient for confirmed diagnosis. Patient was admitted and managed conservatively with oral analgesics. After a few days, patient was discharged in satisfactory condition.
Cutaneous vasculitis, IgM antibodies, Liver function test
Case Report
A seven-year-old male child presented to Paediatric Emergency with complaint of bilateral lower limb swelling and pain in the right knee for the last three days. Patient also has intermittent colicky pain and loss of appetite for the last 5 days. On detailed history taking, it was found that patient had history of jaundice one month back.
Patient was febrile and vitals were normal, Blood Pressure-104/66 mmHg, Pulse Rate-102/min. He had swelling over right knee with reduced range of motion and multiple purple coloured palpable lesions (purpura) ranging from less than 1 cm to at least 3 cm over lower extremities and buttocks. On examination, liver was palpable 3 cm below right costal margin in mid-clavicular line. Rest of the systemic examination was within normal limits. Clinical diagnosis was HSP.
Laboratory evaluation showed haemoglobin 12.0 g/dL, Total Leucocyte Count (TLC) 10,000/mm3, platelets 5,40,000/mm3 (Thrombocytosis). Urine and stool analysis was normal. Following tests were ordered to rule out complications of HSP like anaemia, thrombocytosis, haematuria and occult blood in stool. Ultrasonography of abdomen done to rule out mesenteric ischemia was also normal. C-Reactive Protein (CRP) was negative, Complement component 3 (C3) was normal, Erythrocyte Sedimentation Rate (ESR) 30mm/hr, Aspartate Aminotransferase were 237 IU/L, Alanine Aminotransferase were 219 IU/L and Total Serum Bilirubin of 5.0 mg/dL. Prothrombin Time (PT) and Partial Thromboplastin Time with Kaolin (PTTK) were normal. Renal function tests were normal. Hepatitis B surface Antigen (HBsAg), Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) were negative. HAV IgM Antibodies came out to be positive. HSP (leukocytoclastic vasculitis) is a clinical diagnosis so no differential diagnosis was kept at the initial presentation.
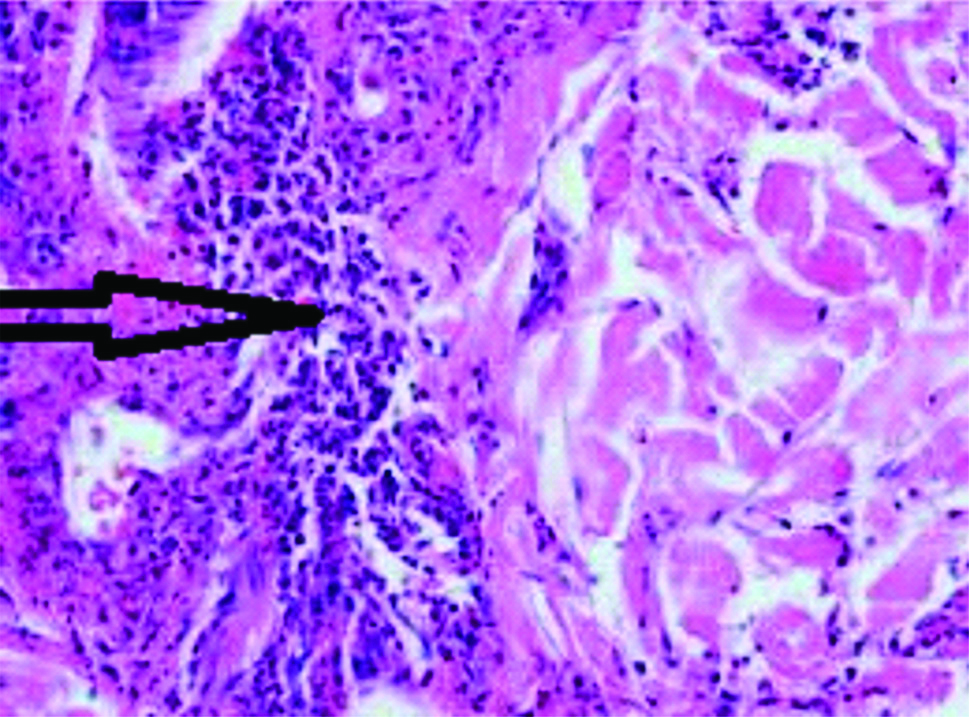
Diagnosis of HSP is mainly clinical but histopathological examination of skin biopsy was also done which was supportive as it also demonstrated leukocytoclastic vasculitis [Table/Fig-1] and IgA deposition. So based on laboratory investigations and histopathological findings, diagnosis of HSP was made.
Leukocytoclastic vasculitis with lymphocytic infiltration with Haematoxylin and Eosin staining in high magnification (45X).
Patient was admitted and managed conservatively with oral analgesics and gradually improved in 4 days. Appetite and jaundice improved. Aspartate Aminotransferase was 98 IU/L, Alanine Aminotransferase was 94 IU/L, Total Serum Bilirubin was 2 mg/dL. Liver regressed and no longer was palpable. Joint swelling also improved and there was no restriction of joint movement.
After a few days, patient was discharged in satisfactory condition with advice to follow-up. Antibodies were not tested on follow-up.
Discussion
HSP is a small vessel arteritis in which there is deposition of IgA immune complexes in the skin and glomeruli [1]. The exact pathogenesis of HSP is unknown, but it usually follows upper respiratory tract infections like streptococci, hepatitis A virus, hepatitis B virus, Coxsackievirus, adenovirus, parvovirus B19, mycoplasma etc. There are also many other associations apart from infections which involves hereditary and antigenic [2,3]. The index case had Hepatitis A infection.
HSP usually present with palpable purpura occurring in lower extremities. Other symptoms include arthritis, arthralgia, abdominal pain, diarrhea, haematuria and malena. Chronic renal disease develops in 1-2% of children with HSP and less than 5% of those develop ESRD [4].
There are studies that showed association of HSP with HBV which suggests the relationship between HBV antigens and Immune Complex-Mediated lesions [5-7]. But, HSP association with hepatitis A infection has been rarely reported. The first case of HSP associated with hepatitis A infection was reported by Garty BZ et al., [8]. In 2001, Bozaykut A et al., reported a case involving children aged 4-24 months who had Acute Infantile Haemorrhagic Oedema (AIHO), which is characterised by purpura, ecchymosis and inflammatory oedema of the face and extremities. Skin biopsy demonstrated leucocytoclastic vasculitis. Laboratory studies showed positive hepatitis A IgM and Ig G antibodies suggesting that AIHO was associated with hepatitis A virus infection [9]. LFTs were normal in these children unlike index patient where LFT was deranged.
There are also various case studies which show association of hepatitis C with HSP [10,11]. Similarly, Islek I et al., reported a case of 13-year-old boy with history of jaundice two and a half months back with repeat admission with complaints of joint pain. He was finally diagnosed with HSP with hepatitis A infection [12]. Unlike this case, index patient had history of jaundice one month back.
According to American Academy of Rheumatology classification [13], out of the four, index patient satisfied three criteria i.e., age of less than 20 years, palpable purpura and histologic findings. He did not have bowel angina, the fourth criteria, hence, did not require glucocorticoids. It has been seen that average age of children reported previously in various case reports has been 10.4 years. No incidence of Hepatitis A in HSP can be inferred as there exists only one reported article [14].
Treatment for HSP is usually supportive. Only few patients need Glucocorticoids in cases of GI or renal involvement [15]. Overall, prognosis is good and most children recover in 2-4 weeks [6].
Conclusion(s)
Association of Hepatitis A infection and HSP has been rarely reported. So, Hepatitis A infection should be kept as a differential in a patient presenting with HSP following jaundice. Adequate hydration and analgesia should be provided.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes (from Parents)
For any images presented appropriate consent has been obtained from the subjects. Yes (from Parents)
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 14, 2020
Manual Googling: Jun 16, 2020
iThenticate Software: Jul 22, 2020 (11%)
[1]. Linskey KR, Kroshinsky D, Mihm MC, Hoang MP, Immunoglobulin-A–associated small-vessel vasculitis: A 10-year experience at the Massachusetts General HospitalJ Am Acad Dermatol 2012 66(5):813-22.10.1016/j.jaad.2011.06.01221798626 [Google Scholar] [CrossRef] [PubMed]
[2]. Rigante D, Castellazzi L, Bosco A, Esposito S, Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura?Autoimmun Rev 2013 12(10):1016-21.10.1016/j.autrev.2013.04.00323684700 [Google Scholar] [CrossRef] [PubMed]
[3]. Harder H, Booken N, Goerdt S, Singer MV, Helicobacter pylori infection and dermatologic diseasesEur J Dermatol 2009 19(5):431-44.10.1684/ejd.2009.073919527988 [Google Scholar] [CrossRef] [PubMed]
[4]. Kliegman, Stanton, St. Geme, Schor, Behrman, Nelson Textbook of Paediatrics 192.1 21st ednElsevier publications:1318-20. [Google Scholar]
[5]. Maggiore G, Martini A, Grifeo S, Giacomo CD, Scotta MS, Hepatitis B virus infection and Schonlein-HenochPurpuraAJDC 1984 138(7):681-82.10.1001/archpedi.1984.021404500630196731386 [Google Scholar] [CrossRef] [PubMed]
[6]. Shin JI, Lee JS, Hepatitis B virus infection and Henoch-Schönlein purpuraJ Dermatol 2007 34(2):15610.1111/j.1346-8138.2006.00240.x17239160 [Google Scholar] [CrossRef] [PubMed]
[7]. McClain DM, Maino K, Dwyer TX, Henoch-Schönlein purpura in an adult Filipino man: A case report and literature reviewCutis 2006 77(4):236-40. [Google Scholar]
[8]. Garty BZ, Danon YL, Nitzan M, Schoenlein Henoch purpura associated with hepatitis A infectionAm J Dis Child 1985 139(6):54710.1001/archpedi.1985.021400800170174003354 [Google Scholar] [CrossRef] [PubMed]
[9]. Bozaykut A, Atay E, Atay Z, Ipek IO, Akin M, Dursun E, Acute infantile hemorrhagic edema associated with hepatitis AAnn Trop Paediatr 2002 22(1):59-61.10.1179/02724930212500017511926052 [Google Scholar] [CrossRef] [PubMed]
[10]. Hou JY, Liu HC, Liang DC, Choi YS, Lin CY, Yeh TC, Henoch-Schönlein purpura and elevated Hepatitis C Virus antibody in a girl with nasopharyngeal diffuse large B-cell lymphomaPediatr Neonatol 2011 52(6):349-52.10.1016/j.pedneo.2011.08.00922192264 [Google Scholar] [CrossRef] [PubMed]
[11]. Madison DL, Allen E, Deodhar A, Morrison L, Henoch-Schönlein purpura: A possible complication of hepatitis C related liver cirrhosisAnn Rheum Dis 2002 61(3):281-82.10.1136/ard.61.3.281-a11830445 [Google Scholar] [CrossRef] [PubMed]
[12]. Islek I, Kalayci AG, Gok F, Muslu A, Henoch-Schönlein purpura associated with hepatitis A infectionPediatr Int 2003 45(1):114-46.10.1046/j.1442-200X.2003.01657.x12654084 [Google Scholar] [CrossRef] [PubMed]
[13]. Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, The American College of Rheumatology, 1990 criteria for the classification of Henoch–Schönlein purpuraArthritis Rheum 1990 33(8):1114-21.10.1002/art.17803308092202310 [Google Scholar] [CrossRef] [PubMed]
[14]. Sasan MS, Doghaee MA, Association of Henoch-Schoenlein purpura with hepatitis AIran J Pediatr 2012 22(4):571-72. [Google Scholar]
[15]. Shin JI, Lee JS, Steroids in Henoch-Schonlein purpura and abdominal painArch Dis Child 2006 91(8):714 [Google Scholar]