FNAC is a widely accepted modality for the early diagnosis of mass lesions. Papanicolaou GN the father of cytopathology established Papanicolaou stain (Pap) in 1942 which he modified in 1954 and again in 1960. Pap is still the most preferred stain by cytopathologists for the excellent morphological features attributed to the prior fixation of smears in ethanol and the multiple colors given to differentiate cells [1]. The other stains used are H&E and MGG. There is always scope for improvement in staining techniques which include the advantages of Pap with lesser turnaround time and expenditure. All these have to be achieved without compromising on the quality of cellular morphology. Ultrafast Papanicolaou stain was introduced by Yang GC and Alvarez II in 1994, as a hybrid of Romanowsky and Pap stain with good quality and lesser time but had limitation as the reagents required were not universally available [2]. In order to overcome this, Kamal MM et al., used MUFP stain with easily available Gill’s Haematoxylin and Eosin Azure which is faster with good cytomorphology and clear background [3]. Pap stain requires ethanol for fixation of smears which is expensive. Tata Memorial Hospital, Mumbai developed REAP using 1% acetic acid instead of ethanol [4]. Objectives of this study were to analyse and compare: 1) QI of five stains namely Pap, H&E, MGG, MUFP and REAP; 2) the advantages and disadvantages of each stain on FNAC of breast and lymph node lesions; 3) their turnaround time; and 4) cost effectiveness. QI is the ratio of actual score obtained to the maximum score possible after adding points given individually for the background of smear, overall staining, preservation of cell morphology, nuclear characteristics, cytoplasmic details and air drying artifact. A study has been reported by the same institution earlier about the efficacy of these five stains in salivary gland and thyroid lesions [5].
Materials and Methods
This was a prospective study, done over a period of one year and 11 months from August 2011 to July 2013, in the Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India. The study had clearance from the Institutional Ethical Committee (KIMS/IEC/D-2/2011) and with the following inclusion and exclusion criteria.
Inclusion criteria: Consecutive FNAC of mass lesions from breast and lymph node were included in this study. Selection of these organs was based on the previous experience of the department as these are the common conditions referred to the lab for FNAC.
Exclusion criteria: FNAC from lesions of other organs and exfoliative cytology such as cervicovaginal smears were excluded. The cases in which the FNAC material from breast and lymph node lesion was inadequate for five staining techniques were also excluded. However, routine cytopathological study was made and diagnosis were given in such cases.
The FNAC procedure was performed with the informed consent of the patient and the clinical and radiological details were collected for each case. FNAC of mass lesions included 44 cases of breast lumps and 36 from lymph nodes comprising the consecutive samples in the study period. For all the 80 cases, a minimum of five smears were made on clean glass slides, of which two smears were fixed in 95% ethanol for a minimum of 15 minutes and submitted for Pap and H&E stain. One smear was fixed in methanol and used for REAP stain as it was found to prevent fading of the smears. The remaining two smears were air dried of which one was stained by MGG and the other smear was rehydrated with normal saline and subsequently fixed in alcoholic formalin for MUFP staining. Procedures for MUFP are shown in the [Table/Fig-1] and for REAP in the [Table/Fig-2] and staining methods used for conventional Pap, H&E and MGG were the routine standard procedures. In MUFP, normal saline rehydrates the cells as the smears are air dried and 95% Ethanol can be kept optional for storage or transporting of the slides to another place [3]. Prefixation in methanol for REAP is required as the smears are not air dried and slides have to be preserved. Blotting was done after each step and then smears are mounted after the dips in xylene with DPX and cover slipped.
Procedure for MUFP stain [5].
| Step no. | Procedure | Time |
|---|
| 1. | Normal saline | 30 seconds |
| 2. | Alcoholic formalin | 10 seconds |
| 3. | Water | 6 slow dips |
| 4. | Gill’s Haematoxylin | 2 slow dips |
| 5. | Water | 6 slow dips |
| 6. | 95% ethanol | 6 slow dips |
| 7. | Eosin azure-50 | 4 slow dips |
| 8. | 95% Ethanol | 6 slow dips |
| 9. | 100% Ethanol | 6 slow dips |
| 10. | Xylene | 10 slow dips |
Procedure for REAP stain [5].
| Step no. | Procedure | Time |
|---|
| 1. | 1% Acetic acid | 10 dips |
| 2. | Harris Haematoxylin, preheated to 60°C | 10 dips |
| 3. | Tap water | 10 dips |
| 4. | 1% Acetic acid | 10 dips |
| 5. | Orange G-6 | 10 dips |
| 6. | 1% acetic acid | 10 dips |
| 7. | EA-50 | 10 dips |
| 8. | 1% acetic acid | 10 dips |
| 9. | Methanol | 10 dips |
| 10. | Xylene | 10 dips |
Smears are fixed in methanol for 30 minutes
After staining and studying each smear, for all the five stains a scoring system was followed and the scores were added to calculate the QI [Table/Fig-3]. The scores are given for each stain with respect to the: 1) background of the smears after staining; 2) overall staining; 3) preservation of cell morphology; 4) nuclear characteristics; 5) cytoplasmic details; and 6) air drying artifacts. The maximum score for each stain after adding the highest scores given to all six parameters was 17 [5].
Scoring system used in assessment of QI for staining [5].
| Parameter | Score |
|---|
| Background |
| Haemorrhagic background | 1 |
| Clean background | 2 |
| Overall staining |
| Poor | 1 |
| Average | 2 |
| Good | 3 |
| Cell morphology |
| Poorly preserved | 1 |
| Moderately preserved | 2 |
| Well preserved | 3 |
| Nuclear characteristics |
| Smudgy chromatin | 1 |
| Moderately crisp chromatin | 2 |
| Crisp chromatin | 3 |
| Cytoplasmic details |
| Unsatisfactory | 1 |
| Suboptimal | 2 |
| Optimal | 3 |
| Air drying artifacts |
| >50% | 1 |
| <50% | 2 |
| 0% | 3 |
Quality Index=Actual score obtained/maximum score possible.
Statistical Analysis
Descriptive and inferential statistical analysis was used and the results on continuous measurements were presented on Mean±SD (Min-Max). The significance of study parameters on categorical scale between two or more groups was assessed by Chi-square/ Fisher’s-exact test. The Statistical software SPSS version 15.0 was used for analysing the results.
Results
FNAC of lymph node lesions were done for patients aged between 11 years to 75 years. Patients presenting with lymph node lesions were younger out of which 4 (11.1%) were in the 2nd decade as compared to the age of patients with breast lesions and 8 (18.2%) of them presented in 3rd decade. Distribution of cases in lymph node was maximum in the age group of 31-40 years with 11 (30.6%) cases and majority of the breast lesions 19 (43.2%) were in the age group of 41-50 years [Table/Fig-4].
Age distribution of the subjects.
| Age in years | Lymph node | Breast |
|---|
| 1-10 | 0 (0%) | 0 (0%) |
| 11-20 | 4 (11.1%) | 0 (0%) |
| 21-30 | 3 (8.3%) | 8 (18.2%) |
| 31-40 | 11 (30.6%) | 12 (27.3%) |
| 41-50 | 9 (25%) | 19 (43.2%) |
| 51-60 | 5 (13.9%) | 3 (6.8%) |
| 61-70 | 3 (8.3%) | 2 (4.5%) |
| 71-80 | 1 (2.8%) | 0 (0%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 42.92±14.69 | 41.41±10.56 |
Lymph Node lesions were more common in males with 21 (58.3%) cases and most of the breast lesions studied was in females with 43 (97.7%) cases [Table/Fig-5].
Gender distribution of the subjects.
| Gender | Lymph Node | Breast |
|---|
| Male | 21 (58.3%) | 1 (2.3%) |
| Female | 15 (41.7%) | 43 (97.7%) |
| Total | 36 (100%) | 44 (100%) |
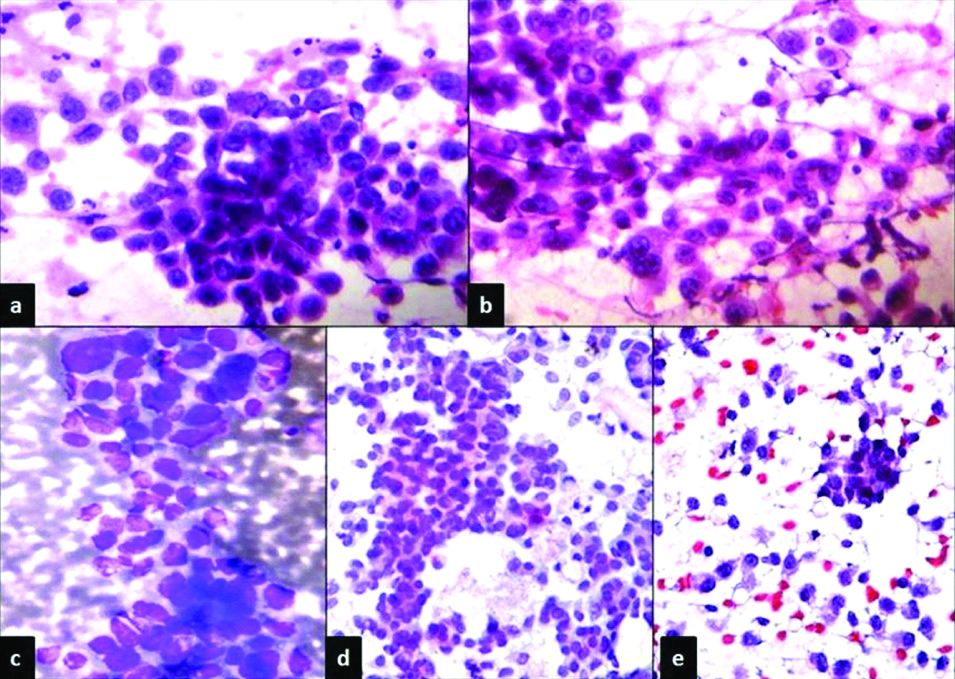
Staining characteristics in breast lesions in all the 44 cases of are shown in the [Table/Fig-6]. The diagnosis included fibroadenoma in 11 (25%), 12 (27.2%) cases of fibrocystic disease, 13 (29.5%) cases of breast carcinoma, 5 (11.4%) cases of acute suppurative process, 2 (4.6%) of granulomatous mastitis and 1 (2.3%) Lactational nodule. FNAC of breast lesions studied with five cytological stains showed the background which was clean in 35 (79.5%) of MUFP and H&E, whereas 38 (86.4%) of MGG smears showed haemorrhagic background. Overall staining was good in 42 (95.5%) with Pap and H&E stains. With REAP 35 (79.5%) cases showed overall good staining of smears as compared to average overall staining in 29 (65.9%) with MGG and 28 (63.6%) with MUFP. Well preserved cell morphology was noted in 43 (97.7%) cases of Pap and H&E, 41 (93.2%) of REAP stained smears. Moderately preserved cell morphology was seen in 24 (54.5%) of MUFP smears. Microscopy of the FNAC smears studied with all five cytochemical stains showed characteristic morphology of benign and malignant lesions along with the features related to all the six parameters used to assess QI of stain. Benign lesions such as fibroadenoma of breast showed cohesive clusters of uniform ductal cells and stromal fragments with scattered prominent myoepithelial cells. Fibrocystic disease showed cyst macrophages in a fluid background with benign ductal cells and suppurative lesions showed inflammatory cells. Smears of malignant lesions such as breast carcinoma showed high degree of cellularity, marked nuclear pleomorphism, abnormal mitoses and necrotic background with the striking absence of myoepithelial cells [Table/Fig-7].
Staining characteristics in breast lesions.
| H&E | Pap | MGG | MUFP | REAP |
|---|
| Background |
| • Haemorrhagic | 9 (20.5%) | 13 (29.5%) | 38 (86.4%) | 9 (20.5%) | 21 (47.7%) |
| • Clean | 35 (79.5%) | 31 (70.5%) | 6 (13.6%) | 35 (79.5%) | 23 (52.3%) |
| Overall staining |
| • Poor | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.3%) | 0 (0%) |
| • Average | 2 (4.5%) | 2 (4.5%) | 29 (65.9%) | 28 (63.6%) | 9 (20.5%) |
| • Good | 42 (95.5%) | 42 (95.5%) | 15 (34.1%) | 15 (34.1%) | 35 (79.5%) |
| Cell morphology |
| • Poorly preserved | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • Moderately preserved | 1 (2.3%) | 1 (2.3%) | 16 (36.4%) | 24 (54.5%) | 3 (6.8%) |
| • Well preserved | 43 (97.7%) | 43 (97.7%) | 28 (63.6%) | 20 (45.5%) | 41 (93.2%) |
| Nuclear characteristics |
| • Smudgy chromatin | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • Mod crisp chromatin | 3 (6.8%) | 1 (2.3%) | 21 (47.7%) | 33 (75%) | 5 (11.4%) |
| • Crisp chromatin | 41 (93.2%) | 43 (97.7%) | 23 (52.3%) | 11 (25%) | 39 (88.6%) |
| Cytoplasmic details |
| • Unsatisfactory | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • Sub-optimal | 2 (4.5%) | 1 (2.3%) | 19 (43.2%) | 27 (61.4%) | 7 (15.9%) |
| • Optimal | 42 (95.5%) | 43 (97.7%) | 25 (56.8%) | 17 (38.6%) | 37 (84.1%) |
| Air drying artifacts |
| • >50% | 0 (0%) | 0 (0%) | 1 (2.3%) | 1 (2.3%) | 0 (0%) |
| • <50% | 15 (34.1%) | 13 (29.5%) | 15 (34.1%) | 18 (40.9%) | 16 (36.4%) |
| • 0% | 29 (65.9%) | 31 (70.5%) | 28 (63.6%) | 25 (56.8%) | 28 (63.6%) |
Cellular smears from carcinoma of breast showing highly pleomorphic cells with absence of myoepithelial cells, stained with: a) Pap stain, X400; b) H&E, X 400;c) MGG, X 400; d) MUFP, X 400; and e) REAP stain, X 400.
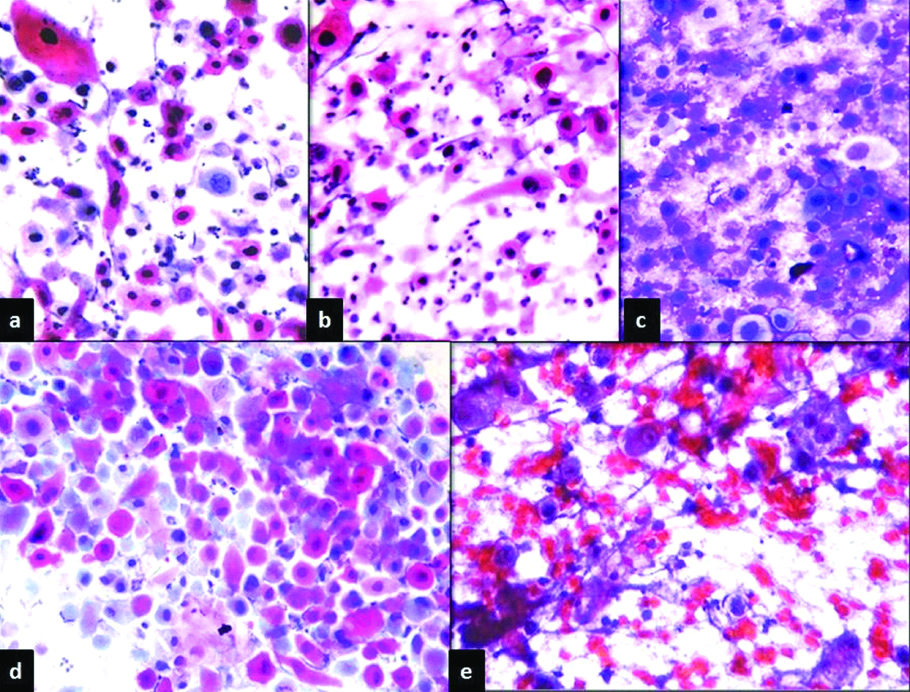
There were 23 (63.9%) out of 36 lesions in lymph nodes were predominantly benign conditions including 14 (38.9%) cases of tuberculous lymphadenitis, 6 (16.7%) reactive lymphadenitis and 3 (8.3%) cases having acute suppurative process. There were 13 (36.1%) cases of malignancy including 4 (11.1%) cases of lymphoma and remaining 9 (25%) were metastatic deposits. The metastatic tumours included 4 (11.1%) cases of Squamous cell carcinoma, 2 (5.6%) cases of poorly differentiated carcinoma and remaining 3 (8.3%) cases were not typed specifically and were reported as ‘smears positive for malignancy. FNAC smears of lymph node lesions yielded staining characteristics as listed according to the parameters for assessment of QI [Table/Fig-8]. The background was clean in 31 (86.1%) of MUFP stained smears. Overall staining was good in 35 (97.2%) with Pap stain, followed by 33 (91.7%) of H&E and 23 (63.9%) of REAP. MUFP stain showed less of air drying artifacts and this stain was very good for all inflammatory lesions of both breast and lymph node lesions. Cytoplasmic details were optimal in 35 (97.2%) with Pap stain, 31 (86.1%) with H&E and air drying artifacts were not seen in 24 (66.7%) with MUFP and 23 (63.9%) with MGG, whereas 19 (52.8%) of H&E and 17 (47.2%) of REAP showed <50% air drying artifacts. Microscopic features of the lymph node aspirates stained with five stains showing features as discussed above are highlighted in the microphotograph [Table/Fig-9] of a case of metastatic deposits of keratinising squamous cell carcinoma.
Staining characteristics in lymph node lesions.
| H&E | Pap | MGG | MUFP | REAP |
|---|
| Background |
| • Haemorrhagic | 12 (33.3%) | 10 (27.8%) | 24 (66.7%) | 5 (13.9%) | 24 (66.7%) |
| • Clean | 24 (66.7%) | 26 (72.2%) | 12 (33.3%) | 31 (86.1%) | 12 (33.3%) |
| Overall staining |
| • Poor | 0 (0%) | 0 (0%) | 0 (0%) | 3 (8.3%) | 0 (0%) |
| • Average | 3 (8.3%) | 1 (2.8%) | 26 (72.2%) | 19 (52.8%) | 13 (36.1%) |
| • Good | 33 (91.7%) | 35 (97.2%) | 10 (27.8%) | 14 (38.9%) | 23 (63.9%) |
| Cell morphology |
| • Poorly preserved | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • Moderately preserved | 4 (11.1%) | 0 (0%) | 11 (30.6%) | 10 (27.8%) | 7 (19.4%) |
| • Well preserved | 32 (88.9%) | 36 (100%) | 25 (69.4%) | 26 (72.2%) | 29 (80.6%) |
| Nuclear characteristics |
| • Smudgy chromatin | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • Mod crisp chromatin | 5 (13.9%) | 0 (0%) | 23 (63.9%) | 24 (66.7%) | 3 (8.3%) |
| • Crisp chromatin | 31 (86.1%) | 36 (100%) | 13 (36.1%) | 12 (33.3%) | 33 (91.7%) |
| Cytoplasmic details |
| • Unsatisfactory | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.8%) | 0 (0%) |
| • Sub-optimal | 5 (13.9%) | 1 (2.8%) | 13 (36.1%) | 12 (33.3%) | 8 (22.2%) |
| • Optimal | 31 (86.1%) | 35 (97.2%) | 23 (63.9%) | 23 (63.9%) | 28 (77.8%) |
| Air drying artifacts |
| • >50% | 0 (0%) | 0 (0%) | 1 (2.8%) | 1 (2.8%) | 1 (2.8%) |
| • <50% | 19 (52.8%) | 16 (44.4%) | 12 (33.3%) | 11 (30.6%) | 17 (47.2%) |
| • 0% | 17 (47.2%) | 20 (55.6%) | 23 (63.9%) | 24 (66.7%) | 18 (50%) |
Smears of lymph node aspirates from metastastic deposits of keratinising squamous cell carcinoma, stained with: a) Pap stain, ×400; b) H&E, ×400; c) MGG, ×400; d) MUFP, ×400; and e) REAP stain, ×400.
Comparative mean QI scores for Pap, H&E, MGG, and MUFP and REAP stains individually for breast and lymph node FNAC smears were calculated after studying all the parameters and assigning scores and adding them [Table/Fig-10]. For H&E Stain, mean QI score difference was statistically significant, with higher score for breast followed by lymph node and with Pap stain similar results were noted. Mean QI for MGG stain was 0.81 for both breast and lymph node lesions. The mean QI for MUFP stain was higher for lymph node followed by breast smears. The mean QI of REAP stain was higher for breast followed by lymph node. Of all the five cytological stains used for lymph node smears, highest QI was noted for Pap stain followed by H&E, REAP, MUFP and MGG. In cases of breast lesions Pap stain showed maximum QI again followed by H&E, REAP, MGG and MUFP [Table/Fig-11].
Comparative mean Quality Index (QI) scores for Pap, H&E, MGG, MUFP and REAP stains individually for breast and lymph node FNAC smears.
| PAP score | Lymph node | Breast |
|---|
| <0.80 | 0 (0%) | 1 (2.3%) |
| 0.81-1.0 | 36 (100%) | 43 (97.7%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 0.95±0.04 | 0.96±0.06 |
| H&E score | Lymph node | Breast |
|---|
| <0.80 | 4 (11.1%) | 1 (2.3%) |
| 0.81-1.0 | 32 (88.9%) | 43 (97.7%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 0.92±0.08 | 0.96±0.05 |
| MGG score | Lymph node | Breast |
|---|
| <0.80 | 14 (38.9%) | 18 (40.9%) |
| 0.81-1.0 | 22 (61.1%) | 26 (59.1%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 0.81±0.09 | 0.81±0.09 |
| MUFP score | Lymph node | Breast |
|---|
| <0.80 | 13 (36.1%) | 26 (59.1%) |
| 0.81-1.0 | 23 (63.9%) | 18 (40.9%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 0.85±0.12 | 0.80±0.12 |
| REAP score | Lymph node | Breast |
|---|
| <0.80 | 7 (19.4%) | 1 (2.3%) |
| 0.81-1.0 | 29 (80.6%) | 43 (97.7%) |
| Total | 36 (100%) | 44 (100%) |
| Mean±SD | 0.88±0.09 | 0.92±0.06 |
Quality Index (QI) of the five stains used for breast and lymph node aspirates.
| Organ | Number of cases | QI of H&E | QI of Pap | QI of MGG | QI of MUFP | QI of REAP |
|---|
| Breast | 44 | 0.94 | 0.96 | 0.81 | 0.80 | 0.92 |
| Lymph Node | 36 | 0.92 | 0.95 | 0.81 | 0.85 | 0.88 |
Discussion
Cytology had its humble beginning with an idea of looking at cells taken from the imprints of cut surface of fresh surgical specimens to obtain instantaneous information [6]. There were four stages with time in which cytopathology became an important diagnostic modality; the first primitive stage (1860-1940), the exfoliative cytology (1940-1960), development as population screening method and FNAC (1955-1985) and present integration with newer technology (from 1985 till date [1]. FNAC was first introduced by Franzen, a haemato-oncologist of Sweden and the technique was further developed by Soderstrom, Fox and Lopes Cardoso, Von Ham, Crepinko and Hauptmann [7,8]. John Webb of UK, a surgeon used the technique and was supported by some of the renowned cytopathologists of that time [9]. Then, it became popular in the USA after sometime since its early use in the 1930s. The focus of FNAC is to procure a satisfactory cell yield. It has many advantages as the technique is relatively painless, gives an early result, has less risk of complications for the patient and also economical [10]. The conventional Pap stain uses ethanol for fixation of smears which is expensive and laboratory requires license for procuring it in large quantity. To avoid this problem REAP, a less expensive and rapid method was tried first by the Department of Pathology of Tata Memorial Hospital, Mumbai, Maharashtra, India. In this method smears are prefixed in methanol and 95% ethanol baths are replaced by 1% acetic acid and the cell morphology is excellent [4].
Till date Pap stain remains the traditional and most preferred stain followed by H&E, not only for the gynaecological cytology but also for FNAC. The stains used for air dried smears, such as Jenner-Giemsa and Diff-Quick methods do not provide good quality. Shinde PB and Pandit AA had developed rapid Pap methods with 4 minutes, 5 minutes and 90 seconds, respectively with much reduced time taken than for conventional Pap stain. These were not satisfactory as the cellular morphology was not well preserved [11]. Yang GC and Alvarez II in 1994 introduced Ultra Fast Papanicolaou (UFP) stain which combined the favourable features of Romanowsky and Pap stain with 90 seconds as the time required for the procedure [2]. The main limitation of UFP stain was that Richard Allan Haematoxylin and Richard Allan cytostain, used were not easily available especially in the developing countries. Kamal MM made two modifications in the UFP stain, the first modification was that instead of Richard Allan Haematoxylin, Gills Haematoxylin was used and the second was replacement of Richard Allan cytostain (alcoholic mixture of orange G, Eosin Y, Light Green and Aniline blue) by modified Eosin Azure an alcoholic mixture of Eosin Y, light green, Phosphotungstic acid and glacial acetic acid. Then onwards this method became more familiar as MUFP stain which overcomes the problem of shortage of ingredients coupled with a short staining time of 130 seconds and good cytomorphology [3]. In this study, a clean background was provided by MUFP instead of a haemorrhagic one in 79.5% of smears as compared to Pap which showed clean background in 70.5% and the least number was with MGG (13.6%) for breast FNAC and the difference was statistically significant with p-value <0.001.
Venkatesh K et al., have reported clean background with MUFP in 80% smears of salivary gland and 76.7% of thyroid lesions, with REAP stain it was 76.7% and 26.7%, respectively [5]. Shinde PB and Pandit AA reported 95% of the smears in MUFP having clean background and studied QI in their study for four sites, lymph node, breast, thyroid and salivary gland [11]. Choudhary P et al., found that MUFP stained smears had clean background and better morphology stating better QI with breast and lymph node lesions [12]. A comparison of QI for MUFP stain made with observations of Shinde PB and Pandit AA and Choudhary P et al., is shown in the [Table/Fig-12], which shows more number of cases are included in the present study [11,12].
Quality Index (QI) of MUFP stain of the present study compared with results of other studies [11,12].
| Shinde PB and Pandit AA (2006) | Choudhary P et al., (2012) | Present study |
|---|
| Organ | Number of cases | QI of MUFP | Number of cases | QI of MUFP | Number of cases | QI of MUFP |
|---|
| Breast | 16 | 0.92 | 23 | 0.97 | 44 | 0.80 |
| Lymph node | 15 | 0.98 | 43 | 0.98 | 36 | 0.85 |
Maruta J et al., reported that MUFP stain lyses red blood cells in the background, makes the smear thinner, clearer and better for observation [13]. Chan JK and Kung IT reported that the rehydration with normal saline restores the transparency of air-dried cells and their nuclear details become better [14]. In addition normal saline lyses the red cells in the aspirate, unmasks tumour cells and the cells appear larger because of air-drying and the nucleoli are more distinct and stain red [15].
The overall staining was good with maximum score for Pap stain and H&E in this study followed by REAP, MUFP and MGG in breast FNAC (p-value of <0.001). This is comparable to the study by Idris AAA and Hussain MS which had maximum score for Pap followed by H&E and MGG. Cell morphology was well preserved, which was maximum with Pap stain (98.4%) followed by H&E (94%), REAP (80.7%), MGG (67.4%) and MUFP (52.7%) and the difference was significant with p-value <0.001 [16]. Nuclear characteristics were best seen with Pap stain followed by H&E, REAP, MGG, and MUFP. The nuclei were graded as having smudgy chromatin, moderately crisp and crisp chromatin. Gupta S et al., showed that crisp nuclear features were seen in 73.3% of REAP stained smears [17].
The cytoplasmic features of breast FNAC are graded as unsatisfactory, suboptimal and optimal. Cytoplasmic features are better and optimal in 97.7% of Pap stained smears followed by H&E, REAP, MGG and MUFP. Biswas RR et al., reported 100 smears out of 110 Pap smears stained with REAP showed optimal cytoplasmic staining [18]. Dighe SB et al., reported that 181 Pap smears out of 200 stained with REAP showed optimal cytoplasmic features [4].
Thakur M and Guttikonda VR studied the quality of MUFP stain in FNAC smears of head and neck swellings with routine Pap, H&E and Giemsa stain. They found that MUFP stain provides suitable alternative with excellent morphological quality and lesser staining time which is reiterated by the present study [19].
Izhar S et al., used REAP on cervical smears and do not recommend this stain for research in tertiary centers where the slides need to be preserved for a long time as the stain fades after 6 months [20]. Sinkar P and Arakeri SU studied MUFP on FNAC smears and reported 91.6% of smears having clean background as compared to 66.4% with conventional Pap [21]. Arul P et al., have reported QI of MUFP stain for breast, thyroid, lymph node, soft tissue, salivary gland, and body fluids as 0.9, 0.93, 0.95, 1, 0.94, and 1.0, respectively and found the stain useful in making easy diagnosis in most of the cases when compared to Pap stain [22]. Shastri SK et al., studied 100 cases of ultrasound guided FNAC for abdominal lesions and found that smears stained with MUFP showed clean background and overall good cellular morphology with better QI than Pap stain [23].
Cytopathology including both exfoliative cytology and FNAC has come a long way. There are a few comparative studies on the QI of cytopathological stains. Pap stain is a better stain when compared to H&E but both of them take more time and expensive than MUFP and REAP. For developing countries, lesser expenditure is also an important factor and there should be no compromise on the quality of staining. Therefore, studies in search of better methods go on and each laboratory develops reliable and convenient techniques of its own for regular use.
Limitation(s)
Cytological study comparing five staining methods, observing six parameters to find QI was difficult to apply for all the cases and therefore only smears of breast and lymph node aspirates were used. Interpretation of cytology smears is highly subjective and there can be differences in opinion. Incorporation of MUFP and REAP in routine cytology needs practice and the efficiency improves with time.
Conclusion(s)
Undoubtedly, conventional Papanicolaou stain is excellent for studying FNAC smears of breast and lymph node lesions with the highest QI and gives best cell morphology except for the presence of air drying artifacts. Next in quality is H&E followed by MGG stain. REAP is as good as Pap with crisp nuclear features. The advantages of MUFP are clean background, less air drying artifacts, lesser time for fixation and better nuclear morphology with red prominent nucleoli. Air drying artifacts are less with MGG and MUFP which are good for lymph node and inflammatory lesions of breast. REAP and MUFP stains have good QI can be used as routine cytological stains as they are economical and have lesser turnaround time.
Smears are fixed in methanol for 30 minutes