Pleural effusions are abnormal collection of fluid in the pleural space, manifestated in pulmonary, pleural or extra-pulmonary disease [1]. Approximately, 0.1 to 0.2 mL/kg of fluid produced in the pleural leaves facilitates the normal pleural movements. In certain clinical conditions there might be imbalance between the production and reabsorption of this fluid which may eventually leads to pleural fluid collection. Mechanisms of pleural effusion includes increased hydrostatic pressure, decreased oncotic pressure and increased permeability in the microvascular circulation in association with increased negative pressure in the pleural space followed by separation of pleural leaves, decrease in lymphatic drainage capacity and transition from the abdomen to the thorax [2,3].
Diagnostic approaches in pleural effusion include, Radiology (conventional radiography, ultrasonography, computersized tomography), Thoracentesis (pleural fluid analysis), closed pleural biopsy and video assisted thoracoscopic biopsy. Currently, Light’s criteria are being used to differentiate pleural fluid into transudates and exudates. However, exudative effusions are further commonly classified into tubercular, parapneumonic and malignant effusions. As of now pleural fluid Adenosine Deaminase (ADA) is being used for differentiating tubercular effusion from other exudative effusions. There is a specified cut-off for ADA (>45 IU) to suggest tubercular effusions [4,5].
The aetiology of effusions varies according to presence or absence of tuberculosis, to achieve a specific diagnosis, more informative tests are required. Biochemical, microbiologic and cytological analyses of pleural effusion are the fundamental studies to determine the aetiology of the effusion but it is not easy to find the main cause every time. Therefore, several biomarkers have been suggested to help in differential diagnosis. Procalcitonin, Amyloid A and CRP are well known acute-phase proteins and the results of these have lately been proposed to use for differentiation of infectious diseases from other origin of pleural effusion [6].
A number of markers are being currently tested, of which pleural fluid CRP levels have gained attention. As of now very few studies have focused on CRP levels in pleural effusion [7-9]. Increased production of this protein is triggered by cytokines, IL6, TNF and IL1, released by inflammatory cells [10]. The current research focused to determine the clinical significance and diagnostic role of pleural fluid CRP level in the aetiological diagnosis of exudative pleural effusion.
Materials and Methods
A cross-sectional study was performed during the period of September 2013 to December 2014 with the prior approval from the Institutional Review Board of Sri Manakula Vinayagar Medical College and Hospital, Puducherry with IEC Code No: 81/2013.
Inclusion criteria: The patients who presented to the pulmonary medicine OPD and were diagnosed with the presence of pulmonary infections associated with acute febrile illness, pulmonary infiltrates, purulent sputum and response to antibiotic treatment; identification of the organism in the pleural fluid; or the presence of emphysema, associated with the finding of frank pus in the pleural cavity with pleural effusion were included in the study.
Exclusion criteria: Patients below 10 years of age, patients in emergency ward, critically ill and patients who were not willing to participate in the study were excluded.
Total 130 patient with the above mentioned inclusion criteria were presented to the hospital during the study period. A sample size of 98 was calculated from the 130 population of patients who were presented to the pulmonary medicine OPD and diagnosed to have pleural effusion were included for the present study, using 5% error margin at 95% confidence interval, assuming 50% of the patients have all the measured outcomes documented in their case files. However, only 53 patients expressed the willingness to participate in study. Written informed consent was taken from the willing patients in their native language (Tamil). A detailed medical history interview and physical examination was done for the recruited study subjects.
The subjects recruited for the study, were worked up for their complete blood count, random blood sugar and ECG. Chest X-ray was done for confirmation of pleural effusion and to rule out co-existing lung conditions; further in few cases USG Thorax was done for confirmation of the pleural effusion. Serum urea and creatinine was done to rule out co-existing kidney diseases, Liver Function Test was done to rule out liver diseases, Echocardiogram was done to rule out cardiac diseases, common cause of transudative effusions. Serum protein and LDH were tested. Sputum AFB, gram stain and culture was done to rule out active tuberculosis and other lung infections. Diagnostic Thoracentesis was done and the pleural fluid was sent for analysis - sugar, protein, Lactic dehydrogenase, ADA, cell count, cytology, AFB and culture. Pleural fluid was subjected for measurement of CRP level by immunoturbidimetric method [11].
Procedure
Thoracentesis was performed after confirming the diagnosis of pleural effusion, the patient was made to sit on the side of the bed with arms and head resting on one or more pillows on the bedside table. Thoracentesis was performed posteriorly after cleaning with an antiseptic solution, several inches from the spine, where the ribs were easily palpated. The rationale for this location was that the arteries, veins and nerves run just inferior to the ribs. The skin was anaesthetised using a short 25-gauge needle by injecting lidocaine, the small needle was then replaced by a 1.5-inch long 22-gauge needle superior to the rib. Aspiration of the pleural fluid was done by slowly piercing the needle towards the pleural space; while clotting was prevented by attaching 50- to 60-mL syringe containing 1 mL of heparin to the pleural space. Clotting makes it difficult to obtain differential white blood cell counts or pH determinations of the pleural fluid. The aspiration procedure continued until the syringe was completely filled. Diagnostic Thoracentesis can be done by commercially available kits [12].
Estimation of Adenosine Deaminase (ADA)
Pleural fluid was subjected to the analysis of ADA MTB by the colorimetric method using standard procedure. The principle involved in this analysis is hydrolyses of ADA to ammonia and inosine. The ammonia on reaction with phenol and hypochlorite in an alkaline medium forms a blue indophenol complex; the intensity of colour is directly proportional to the amount of ADA present in the sample which was measured for the absorbance against the blank [13-16].
Estimation of C-Reactive Protein (CRP)
Pleural fluid CRP was assessed with CRP-Turbilatex-Quantitative turbidimetric immunoassay method which is based on the principle agglutination reaction. The pleural fluid (test specimen) containing CRP forms agglutinin when mixed with latex particles coated with specific anti-human CRP. This reaction results in change in absorbance (540 nm) which is dependent on CRP levels and can be quantified by comparison with a known calibrated CRP concentration [17].
Diagnosis of Parapneumonic Effusion
A diagnosis of parapneumonic effusion was made in the patients with clinicoradiological/ microbiological evidence of pneumonia complicated with pleural effusion.
A diagnosis of tubercular effusion was made if one of the following was present: microbiological evidence of tuberculosis (AFB stain or culture positive) or histopathological/cytological evidence of granuloma suggestive of tuberculosis or a strong clinical suspicion of TB along with high pleural fluid ADA levels.
The diagnosis for the malignant pleural effusion was made if one of the following was present, Pleural fluid cytology positive for malignancy or Pleural biopsy suggestive of malignancy or no cause attributable to development of pleural effusion with evidence of proven malignancy elsewhere. The patients were treated according to the diagnosis [18,19].
Statistical Analysis
Data were analysed using simple descriptive statistics, average, mean and standard deviation. The data was entered and analysed with Epi info software version 3.4.3. ANOVA was utilised for comparing the pleural fluid CRP levels in differentiating transudates and exudates; also in differentiating exudative effusions, where a p-value of < 0.001 was considered statistically significant. The ROC curve was plotted against CRP levels and tubercular/other exudative effusions to illustrate the diagnostic ability.
Results
A total of 53 patients (n=53), diagnosed with pleural effusion, 11 patients had transudative pleural effusions and 42 patients had exudative pleural effusions. Among the exudative effusions (n=42), tuberculous effusions were 26, malignant effusions were 9 and parapneumonic effusions were 7. Among the transudative effusions (n=11), congestive cardiac failure were 5, Chronic Kidney Disease were 5 and alcoholic liver disease was 1. Most of the cases (50.9%) were in the age group between 30 to 50 years. Most of the cases affected with pleural effusion were males, 84.9% (45 cases) and females were 15.1% (8 cases) [Table/Fig-1].
Age and gender distribution of pleural effusion cases.
| Age group (years) | Frequency | Percentage (%) |
|---|
| 10-20 | 2 | 3.8 |
| 20-30 | 7 | 13.2 |
| 30-40 | 12 | 22.6 |
| 40-50 | 15 | 28.3 |
| 50-60 | 7 | 13.2 |
| 60-70 | 8 | 15.1 |
| 70-80 | 1 | 1.9 |
| 80-90 | 1 | 1.9 |
| Gender |
| Male | 45 | 84.9 |
| Female | 8 | 15.1 |
| Total | 53 | 100 |
The common cause of exudative effusion was tuberculosis 61.90%, followed by malignancy 21.40% and parapneumonic effusion 16.70% [Table/Fig-2].
Frequency distribution of the type of pleural effusions and its causes.
| Types of pleural effusion | Frequency | Percentage (%) |
|---|
| Transudates | 11 | 20.8 |
| Exudates | 42 | 79.2 |
| Total | 53 | 100 |
| Causes of transudative effusions |
| Disease | Frequency | Percentage (%) |
| Adrenoleukodystrophy | 1 | 9.0 |
| Congestive cardiac failure | 5 | 45.5 |
| Chronic kidney disease | 5 | 45.5 |
| Total | 11 | 100 |
| Causes of exudative effusions |
| Malignancy | 9 | 21.4 |
| Parapneumonic effusions | 7 | 16.7 |
| Pulmonary tuberculosis | 26 | 61.9 |
| Total | 42 | 100 |
| Causes of pleural effusions (both transudative and exudative effusions) |
| Adrenoleukodystrophy | 1 | 1.9 |
| Congestive cardiac failure | 5 | 9.4 |
| Chronic kidney disease | 5 | 9.4 |
| Malignancy | 9 | 17.0 |
| Parapneumonic effusions | 7 | 13.2 |
| Pulmonary tuberculosis | 26 | 49.1 |
| Total | 53 | 100 |
The average Pleural fluid ADA was 59.46±42.43 and CRP was 35.23±21.07 in pleural effusion, whereas ADA and CRP in Transudates is 16.45±3.10 and 7.37±1.55, followed by ADA and CRP in exudates are, 70.7±4.0 and 42.5±1.7 respectively [Table/Fig-3].
Mean pleural fluid ADA and CRP.
| Variable | Mean±SD |
|---|
| Pleural fluid ADA and CRP in pleural effusion (Transudates and Exudates) | ADA | 59.46±42.43 |
| CRP | 35.23±21.07 |
| Pleural fluid ADA and CRP in transudates | ADA | 16.45±3.10 |
| CRP | 7.37±1.55 |
| Pleural fluid ADA and CRP in exudates | ADA | 70.7±4.0 |
| CRP | 42.5±1.7 |
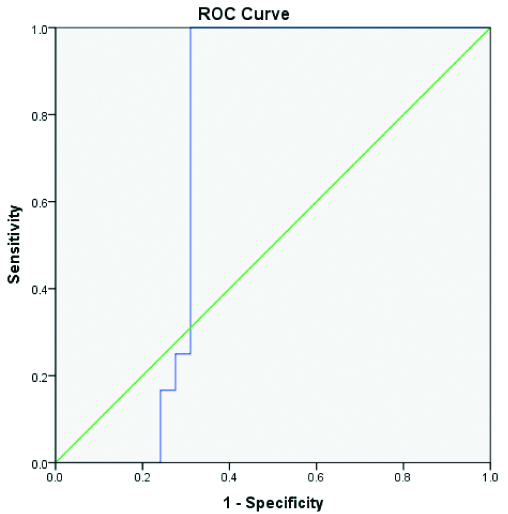
The prevalence of tuberculous effusion was 49.1% in our study. Among the exudative effusions pleural fluid ADA was more than 40 IU/L in tuberculous effusion and a value of less than 40 IU/L excluded the diagnosis of tuberculosis. The mean pleural fluid ADA value was 92.6 for tuberculosis, 28.6 for malignancy and 33.1 for parapneumonic effusions. The mean value of CRP in transudates was 7.37 mg/dL and 42.52 mg/dL in exudates. The mean pleural fluid CRP was 37.5 mg/dL in tuberculosis, 28.2 mg/dL in malignancy and 79.2 mg/dL in parapneumonic effusions [Table/Fig-4]. In our study, the pleural fluid CRP is statistically a significant (p<0.001) marker to differentiate exudative effusions with CRP-value <30 suggestive of malignancy, CRP-value 30-50 mg/L suggestive of tuberculosis and CRP-value >70 mg/L suggestive of parapneumonic effusions. The Pleural fluid CRP is a statistically significant marker in differentiating tubercular effusion from nontubercular exudative effusions with good sensitivity (96.15%), specificity (100%), Positive Predictive Value (PPV) of 100% and Negative Predictive Value (NPV) of 94.12% [Table/Fig-5,6].
Common cause of exudative pleural effusion.
| Pleural fluid | | | N | Mean | SE | p-value |
|---|
| ADA in exudates effusions | Exudates | PTB | 26 | 92.6 | 7.1 | 0.001 |
| MAL | 9 | 28.6 | 3.1 |
| PPE | 7 | 33.1 | 2.5 |
| CRP in exudative effusions | Exudates | PTB | 26 | 37.5 | 0.62 | 0.001 |
| MAL | 9 | 28.2 | 0.79 |
| PPE | 7 | 79.2 | 1.6 |
| CRP among transudates and exudates | Pleural effusion | Transudates | 11 | 7.37 | 1.55 | 0.001 |
| Exudates | 42 | 42.52 | 17.31 |
p-value <0.05 was considered statistically significant (ANOVA was used for comparing three means values ie., Exudative effusion-tuberculosis, Malignancy and Parapneumonic and Student ‘t’ test was used for comparing two mean values ie., Transudates and Exudates).
PTB: Pulmonary tuberculosis; MAL: Malignancy; PPE: Parapneumonic effusion
Test for diagnostic significance of Pleural fluid CRP.
| ADA | |
|---|
| | Positive | Negative | Total |
|---|
| CRP | Positive | 25 | 0 | 25 |
| Negative | 1 | 16 | 17 |
| Total | 26 | 16 | 42 |
Sensitivity: 96.15% Positive predictive value (PPV)- 100%; Specificity: 100% Negative predictive value (NPV)- 94.12%
ROC Graph to illustrate specificity and sensitivity of pleural CRP test.
Discussion
In present study most of the pleural effusion cases in the study were in the age group between 30 to 50 years (50.9%). Males were predominantly affected with 45 cases (84.9%) while that of females were only 8 cases (15.10%). Similar to our study, Khan Y et al., study proved that in 100 patients of pleural effusion; males were predominantly affected with 60% (60 cases) and 40% (40 cases) were women [19]. The reports on pleural effusions of Mehta AA et al., proved that men were predominantly involved with pleural effusions than female.
Among the 53 patients in the study, 42 had exudative effusion (79.20%) and 11 had transudative effusion (20.80%). The common cause of exudative effusion was tuberculosis 61.90%, followed by malignancy 21.40% and parapneumonic effusion 16.70%. The mean pleural fluid ADA value in tuberculosis was 92.6 IU/L, 28.6 IU/L for malignancy and 33.1 IU/L for parapneumonic effusions. A study by Patel AK and Choudhury S reported that the pleural fluid protein was used in differentiating tansudates and exudates with a cut-off value of 3 g/dL [21]. Similar result was found in a study by Gabhale SD et al., where the mean pleural protein was 5.4±1.1 in tubercular effusion, 4.6±1.1 in malignant effusion and 4.0±0.4 in parapneumonic effusion [22]. Tuberculous pleural effusion incidence in developing countries is 18.2-62/100,000 and in the western countries it is 0.42-0.77/100,000 [23]. The most common cause of exudative effusions in United States is parapneumonic effusion followed by malignancy [24].
The pleural fluid ADA levels were higher in tuberculous effusions compared to the nontuberculous exudative effusions in the present study. Among the exudative effusions pleural fluid ADA was more than 40 IU/L in tuberculous effusion and a value of less than 40 IU/L excluded the diagnosis of tuberculosis. In a study done by Tay TR and Tee A of 160 patients with pleural effusion, the pleural fluid ADA level was higher in tuberculous effusions compared to nontuberculous effusions, similar to present study [25].
In present study pleural fluid ADA cut-off value for diagnosis of tuberculosis was more than 40 IU/L, similar to the study by Mathur PC et al., where pleural fluid ADA more than 40 IU/L was the cut-off value for diagnosis of tuberculous effusions [26].
In the present study, the pleural fluid CRP level was higher in parapneumonic effusions than the tuberculous effusions, with a mean CRP-value of 79.2 in parapneumonic effusion and 37.5 in tuberculous effusion. Gabhale SD et al., studied 187 patients with exudative pleural effusions, the pleural fluid CRP level was higher in parapneumonic effusions than the tuberculous effusions similar to the current study, with a mean value of 134.03 in parapneumonic and 66.54 in tuberculous effusions [22].
The pleural fluid CRP level was useful in differentiating exudative pleural effusions. The exudative effusion was sub grouped into tuberculous, malignant and parapneumonic effusions. Pleural fluid CRP-value in tuberculous effusion was more than 30 mg/L, while that of malignant effusion it was less than 30 mg/L. Similar result was found in a study done by Perlat K et al., where pleural CRP levels were below 30 mg/L in an exudative effusion strongly which suggests malignancy while levels above 30 mg/L were suggestive of inflammatory aetiology [27].
In the present study, pleural fluid CRP cut-off value in parapneumonic effusions was >70 mg/l, similar to a study by Gabhale SD et al., with 187 patients of exudative pleural effusions, where the pleural fluid CRP cut-off value in parapneumonic was ≥90 mg/l [22]. The pleural fluid CRP levels with cut-off value of 30 mg/l was useful in differentiating tuberculous effusion from malignant effusions. Similar result was found in a study by Ji Q et al., with 209 patients of pleural effusions in which the pleural fluid CRP levels with cut-off value of 35.2 mg/l was useful in differentiating malignant effusions from infective effusions [28]. The strength of the present study was varied diagnosis of exudative effusions and accurate measurement of CRP levels along with comparison of a well-established marker. Large scale studies are required in the future to establish a definite role of CRP in exudative pleural effusions.
Limitation(s)
The study population was predominantly tubercular as its prevalence is more in the present geographical location. In future more studies with larger sample size having adequate parapneumonic and malignant effusions will have greater impact on pleural fluid CRP levels as a significant marker in differentiating tubercular from nontubercular effusions.
Conclusion(s)
Pleural fluid CRP is a useful diagnostic marker for differentiating exudative and transudative effusions. Also, Pleural fluid CRP is a statistically significant marker in differentiating tubercular effusions from nontubercular exudative effusions. From the present study, it is evident that pleural fluid CRP determination is a simple and rapid method for differentiating exudative pleural effusions.
p-value <0.05 was considered statistically significant (ANOVA was used for comparing three means values ie., Exudative effusion-tuberculosis, Malignancy and Parapneumonic and Student ‘t’ test was used for comparing two mean values ie., Transudates and Exudates).
PTB: Pulmonary tuberculosis; MAL: Malignancy; PPE: Parapneumonic effusion
Sensitivity: 96.15% Positive predictive value (PPV)- 100%; Specificity: 100% Negative predictive value (NPV)- 94.12%