Dermatophytosis or tinea or ringworm are caused by a group of closely related fungi that invade the keratinised tissues of skin, hair and nails. These fungi are called dermatophytes which are classified into three genera: 1) Trichophyton; 2) Epidermophyton; and 3) Microsporum. Dermatophytosis remained a public health problem. According to WHO, the prevalence rate of superficial mycotic infection worldwide has been found to be 20-25% [1]. The estimated life risk of acquiring tinea infection is 10-20% [2]. The distribution of the dermatophytosis and their aetiological agents vary from one ecological niche to another and depends on several factors, such as lifestyle, socio-economic status, occupation, and climatic conditions, therefore some species are widely distributed whereas others are geographically restricted [3]. Recent surge in mycotic infections have been noted which may be related to inappropriate use of antibiotics, immunosuppressive conditions like HIV and malignancy and immunosuppressive drugs commonly used in organ transplantation recipients [4].
A particular dermatophyte species may produce lesion at multiple anatomic sites. Moreover, clinically similar lesions may be produced by different species [5]. It is now well recognised that appropriate mycological diagnosis of clinically suspected cases of dermatophytosis is essential before initiation of antifungal therapy. Identification of the dermatophyte up to the species level helps in epidemiological assessment as well as guide in therapy, particularly when long duration treatment is planned [6].
Considering this, present study was planned to evaluate the clinical-mycological profile of clinically suspected cases of dermatophytosis prevalent in Saurashtra region of Gujarat, India.
Materials and Methods
This was a cross-sectional observational study conducted in Department of Microbiology at MP Shah Medical College and GG Hospital, Jamnagar, Gujarat, India. After getting a due permission from Institutional Ethical Committee, study was conducted during a period of two years from December 2007 to October 2009.
Inclusion criteria: Two hundred consecutive patients presenting to the dermatology Out Patients Department who had been suspected for tinea infection were enrolled. Presenting symptoms were mostly dry, erythematous, scaly cutaneous lesions or deformed friable, discoloured nails or thick scales in the hair follicles.
Exclusion criteria: Patients of tinea already receiving antifungal treatment were excluded from the study. Written informed consent was taken from every participant. Detailed clinical history of patients was obtained (age, sex, site, type and duration of lesion, predisposing factor like occupational exposure to animal source or family contact were noted). Patients were classified in different clinical types depending on site of the lesion e.g., tinea corporis (body), tinea unguium (nail) etc.
Sample Collection
Samples (either skin scraping, hair or nail) were collected depending upon the site of lesion.
Skin scraping: The affected area was swabbed with 70% alcohol and allowed to dry (to remove contaminant dirt and bacteria). Specimen was collected by scrapping the active margin of the lesion with blunt edge of sterile scalpel.
Hair: Hair was plucked with sterile forceps. Care was taken to obtained basal portion of the hair where fungus is usually found.
Nail: The affected area was cleansed with 70% alcohol and the nail clippings and scrapings beneath the nail was collected.
These samples were then screened for the presence of fungal element by 10% KOH. Nail clippings were immersed in 10% KOH overnight and examined next morning. KOH wet mount was screened for the presence of filamentous septate, branched hyphae, arthrospores. Type and arrangement of spore was noticed to name it as ectothrix based, or endothrix type of infection in case of hair sample [4].
Culture was done on SDA and CMA. SDA with antibiotics (Chloramphenicol and Gentamicin) was used for primary isolation and CMA was used for macroconidia, microconidia and pigment production. After inoculation using scalpel, primary culture on SDA was done and incubated at three different temperatures i.e., 25°C, 30°C and 37°C for four weeks. All cultures were examined bi-weekly for growth and incubated for four weeks before declaring them negative. CMA was used to differentiate Trichophyton rubrum from Trichophyton mentagrophytes based on pigment production on the media. In addition, hair perforation studies were carried out to distinguish between these two species [7,8]. Identification of the organisms were done by growth of fungal colony on culture plate (colour, growth, pigment, reverse pigment), and microscopic appearance of organism (size, shape and arrangement of microconidia and macroconidia, special hyphae and chlamydospore) by using Lactophenol Cotton Blue (LCB) and slide culture method [4,7,8].
Candida albicans was identified by Gram stain (budding yeast cells and psuedohyphae), 10% KOH preparation, and culture characteristic and by performing germ tube and chlamydospore formation. Fusarium spp. was identified by 10% KOH preparation, culture characteristic and by LCB preparation (multiseptate, sickle shaped macroconidia) [4].
Statistical Analysis
Microsoft office 2010 was used to make tables. Descriptive statistics like mean and percentages were used to infer results.
Results
A total of 200 samples were collected from patients with clinically suspected tinea infection out of which 157 were from skin, 23 from nails and 20 were hair samples. Out of them 177 (88.5%) samples were positive by KOH mount [Table/Fig-1] and 127 (63.5%) showed culture positivity [Table/Fig-2].
KOH mount (40X) of skin scrappings showing branching hyaline septate hyphae.
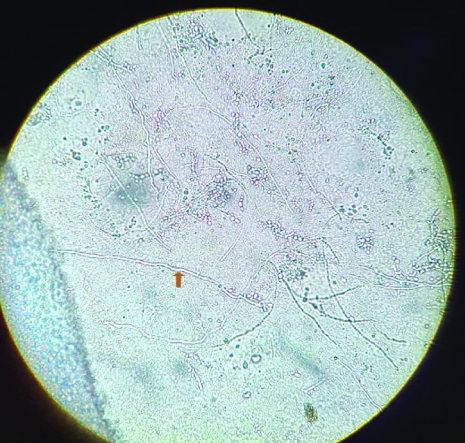
KOH and culture analysis of clinical specimen of dermatophytes.
| Site | No. of cases | KOH +VE only (%) | Culture +VE only (%) | Both culture and KOH +VE (%) | Culture positive (%) |
|---|
| Skin | 157 | 44 (28) | 06 (3.8) | 93 (59.2) | 99 (63) |
| Nail | 23 | 08 (34.7) | 00 (0) | 14 (60.8) | 14 (61) |
| Hair | 20 | 06 (30) | 02 (10) | 12 (60) | 14 (70) |
| Total | 200 | 58 (29) | 8 (4) | 119 (59.5) | 127 (63.5) |
Sample analysis shows that most common age group was 21-30 years (46.5%) followed by 31-40% (23.5%) with mean of 28 years. Male: female ratio was 3:2 [Table/Fig-3]. The samples were further analysed depending upon the clinical manifestations and it was found that 110 cases out of 200 had Tinea corporis (55%), 35 had Tinea cruris (17.5%) and 20 had Tinea capitis (10%). In gender wise correlation of clinical presentation, among males 69 had Tinea corporis, 26 had Tinea cruris, 13 had Tinea capitis, 4 had Tinea pedis, 3 had Tinea unguium. Among female, 41 had Tinea corporis, 9 had Tinea cruris, 7 had Tinea capitis and 6 had Tinea unguium. Therefore, in both males and females Tinea corporis was the commonest lesion followed by Tinea cruris [Table/Fig-3]. The most common group infected with dermatophytosis were agricultural workers (56.69%) followed by labourers (31.49%) [Table/Fig-4].
Age and sex distribution of various clinical types of dermatophytes.
| Clinical types | Age group (years) | Gender | Total cases |
|---|
| 0-10 | 11-20 | 21-30 | 31-40 | 41-50 | 51-60 | >60 | Male | Female | No. | % |
|---|
| Tinea corporis | 2 | 5 | 60 | 26 | 10 | 5 | 2 | 69 | 41 | 110 | 55 |
| Tinea cruris | - | 7 | 21 | 5 | 2 | - | - | 26 | 09 | 35 | 17.5 |
| Tinea capitis | 11 | 5 | 4 | - | - | - | - | 13 | 07 | 20 | 10 |
| Tinea pedis | - | - | 2 | 4 | 1 | - | - | 04 | 03 | 07 | 3.5 |
| Tinea mannum | - | - | - | 3 | 1 | - | - | 02 | 02 | 04 | 2 |
| Tinea unguium | - | - | 3 | 4 | 2 | - | - | 03 | 06 | 09 | 4.5 |
| Tinea barbea | - | - | - | 1 | - | | | 01 | - | 01 | 0.5 |
| Non dermatophyte onychomycosis | - | 1 | 3 | 4 | 3 | 3 | - | 04 | 10 | 14 | 7 |
| Total | 13 | 18 | 93 | 47 | 19 | 08 | 02 | 122 | 78 | 200 | 100 |
| Total % | 6.5 | 9 | 46.5 | 23.5 | 9.5 | 4 | 1 | 61 | 39 | | |
Correlation between occupation and dermatophytosis.
| Occupation | No. of cases | % |
|---|
| Agriculture | 114 | 56.69 |
| Labourer | 62 | 31.49 |
| House wives | 19 | 9.44 |
| Family contact | 5 | 2.36 |
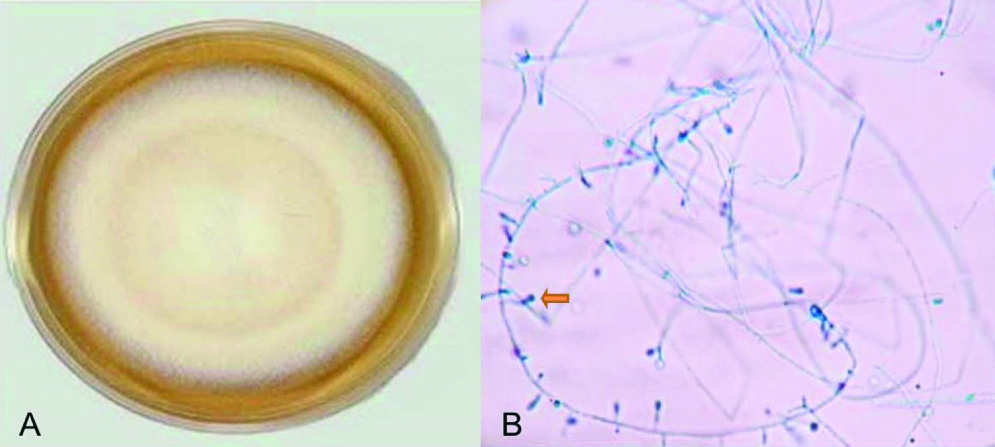
On analysing the 127 dermatophyte species isolates 64 cultures were T.rubrum (50.39%), 18 isolates were T. mentagrophytes (14.17%), 16 isolates were E. flocosum (12.59%) and 10 were T. tonsurans (7.87%). Most common isolate from hair was T.tonsurans while from nail and skin was T. rubrum. All the three cases of Fusarium were isolated form nails [Table/Fig-5]. It was seen that Tinea corporis and Tinea unguium are predominantly caused by T.rubrum. All Tinea pedis cases was caused by T. rubrum. T.tonsurans was predominantly involved in Tinea capitis and E.floccosum in Tinea cruris [Table/Fig-6].
Incidence of different species of dermatophytes and its isolation from different clinical samples.
| Species | No. of cases (%) | Hair | Nail | Skin |
|---|
| T. rubrum | 64 (50.39) | 4 | 6 | 54 |
| T.mentagrophytes | 18 (14.17) | - | 1 | 17 |
| T.tonsurans | 10 (7.87) | 8 | - | 2 |
| M.canis | 05 (3.93) | - | - | 5 |
| M.gypseum | 07 (5.51) | 2 | - | 5 |
| E.flocosum | 16 (12.59) | - | 2 | 14 |
| Candida albicans | 04 (3.14) | - | 2 | 2 |
| Fusarium | 03 (2.36) | - | 3 | 0 |
| Total | 127 | 14 | 14 | 99 |
Clinical type in relation to aetiological species.
| Clinical types | Culture +VE | T. rubrum | T. mentagrophytes | M. canis | M. gypseum | E. floccosum | T. tonsurans | Candida | Fusarium |
|---|
| Tinea corporis | 65 | 42 | 08 | 04 | 04 | 05 | 02 | - | - |
| Tinea cruris | 27 | 10 | 09 | - | - | 08 | - | - | - |
| Tinea capitis | 14 | 01 | 01 | 01 | 03 | - | 08 | - | - |
| Tinea unguium | 07 | 05 | - | - | - | 02 | - | - | - |
| Tinea mannum | 02 | 01 | - | - | - | 01 | - | - | - |
| Tinea pedis | 04 | 04 | - | - | - | - | - | - | - |
| Tinea barbae | 01 | 01 | - | - | - | - | - | - | - |
| Non dermatophyte onychomycosis | 07 | - | - | - | - | - | | 04 | 03 |
| Total | 127 | 64 | 18 | 05 | 07 | 16 | 10 | 04 | 03 |
Discussion
Dermatophytosis has a wide geographical distribution and its incidence varies from place to place. India is a tropical country with hot and humid climatic condition which is considered conducive for dermatophytosis. Other factors like socio-economic condition, occupation and population density also influence its prevalence [9]. This study focused on identifying the demographic distribution, clinical subtypes of suspected cases and identification of the species in confirmed cases of dermatophytosis.
In the present study, it was observed that 46.5% cases of dermatophytosis were in the age group 21-30 years while 23.5% cases were in the age group 31-40 years. Study by Dhayagude S et al., also showed that the common age group involved in dermatophytosis was 21-40 years [9]. The present observation correlates with previous publications by Phudang RT et al., and Konda C et al., [10,11]. However in the study by Sudha M et al., commonest (40.76%) age group was between 30-40 years [12]. Adults in the age group of 20 -40 years are most physically active resulting in increased perspiration. It produces a hot, humid, environment in the body, favouring the growth of dermatophytes [9,12].
The male: female ratio was 3:2 which correlates with other studies by Dhayagude S et al., Sudha M et al., and Doddamani PV et al., [9,12,13]. Higher incidence in males might be due to greater physical and outdoor activity. In the present study, 88% cases were agricultural workers and labourers working outdoors leading to profuse sweating which in turn resulted in increased dermatophyte infection.
A comparison of the direct microscopy and culture results showed that direct KOH mount is good screening test for dermatophytosis because 88.5% samples were positive in KOH mount while 63.5% were positive in culture. Similar results were found in the study by Sudha M et al., where KOH positivity was 86% and culture positivity was 77% [12]. However, in the study by Doddomani PV et al., KOH positivity was 65% while culture positivity was 48% [13].
Culture positivity was highest in hair (70%) followed by skin (63%) and nails (61%) [Table/Fig-2]. In the study by Doddamani PV et al., also culture positivity was highest in hair (100%) followed by skin (50%) and nails (15.7%) [13].
Tinea corporis was the commonest presentation (55%) followed by Tinea cruris (17.5%) which correlates with study by Sudha M et al., who also found Tinea corporis in 56.9% cases and Tinea cruris in 28.5% cases [12]. High incidence of these two types could be due to the severe itching associated with these two conditions, making them seek medical advice. Secondly, trunk including axilla and groin are usually covered with clothes, leading to increased sweating, creating an environment favourable for the dermatophytes.
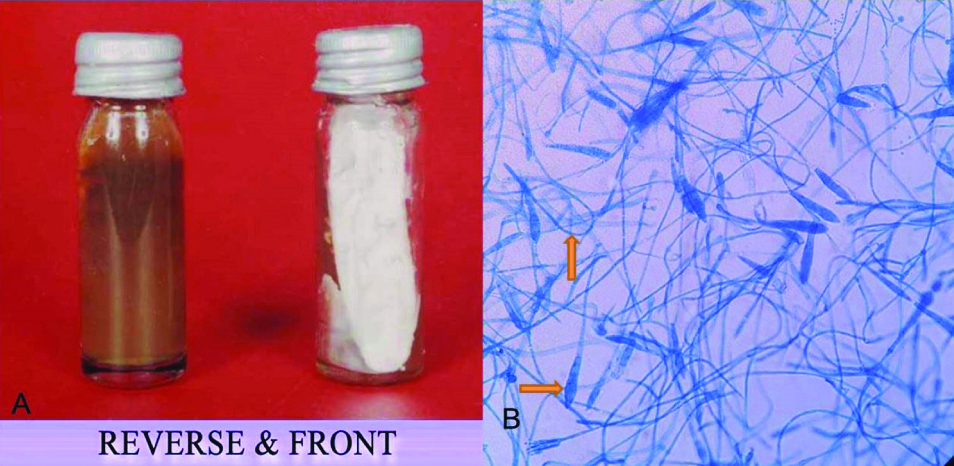
T. rubrum was the predominant (50.39%) isolate followed by T. mentagrophytes (14.17%) [Table/Fig-7], E. floccosum (12.59%) and T.tonsurans (7.87%) [Table/Fig-8] among all culture confirmed cases of dermatophytosis [Table/Fig-5]. Studies by Dhayagude S et al., Sudha M et al., Doddamani PV et al., Dukare A et al., and Jain N et al., also found the T. rubrum as the most common isolate [9,12-15]. T. rubrum may have greater adaptability to survive in varying climatic condition, overcrowding and unhygienic conditions [15]. However Guruprasad KY et al., and Phudang RT et al., found T. mentagrophytes as the most common species [Table/Fig-9] [9-13,15-18].
a) T. mentagrophyte growth in SDA showing pale yellow pigment on back and white powdery colony on front; b) Microscopic examination: LPCB mount (X40) showing cylindrical macroconidia with spiral.
a) T.tonsurans growth in SDA showing velvety, powdery to cream colony with central furrows; b) Microscopic examination: LPCB mount (X 40) showing “balloon” microconidia and stalked microconidia born on short branches.
Comparison of present study with similar other Indian studies (2013-19) [9-13,15-18].
| Author, Year [ref] | M:F | Most common age group (years) | KOH positivity (%) | Culture positivity (%) | Most common clinical type | Most common species |
|---|
| Dhayagude S et al., 2019 [9] | 2.5:1 | 21-40 | 40.3 | 29.4 | Mixed Tinea cruris and corporis | T. rubrum |
| Phudang RT et al., 2019 [10] | 1:1 | 21-30 | 60 | 45 | Tinea unguium | T. mentagrophytes |
| Konda C et al., 2017 [11] | 1.43:1 | 21-30 | 51 | 40 | Tinea corporis | T. tonsurans |
| Sudha M et al., 2016 [12] | 1.65:1 | 31-40 | 86 | 77 | Tinea corporis | T. rubrum |
| Doddamani PV et al., 2013 [13] | 2.7:1 | 21-30 | 65 | 48 | Tinea corporis | T. rubrum |
| Jain N et al., 2014 [15] | 3:1 | 21-30 | 75.5 | 81.6 | Tinea corporis | T. rubrum |
| Guruprasad KY et al., 2019 [16] | 7:3 | 21-40 | 69.6 | 40 | Tinea cruris | T. mentagrophytes |
| Rathoriya SG et al., 2018 [17] | 1.58:1 | 21-30 | 89.3 | 46 | Tinea corporis | T. rubrum |
| Poluri LV et al., 2015 [18] | 2:1 | 21-40 | 58.18 | 56.36 | Tinea corporis | T. rubrum |
| Present study | 3:2 | 21-30 | 88.5 | 63.5 | Tinea corporis | T. rubrum |
Thus, the present study gave valuable insight regarding the prevalence of dermatophytosis in this tertiary care public hospital in Saurashtra region. It is advantageous to use both direct microscopy by KOH and culture for diagnosis in clinical practice as when used alone either may give false negative results. Personal hygiene, socio-economic status, occupation correlates with increased incidence of these infections.
Limitation(s)
Limitation of the present study was that sample population was limited to a single tertiary care government hospital. So, a multicentric study involving private and public centre hospitals would give a better insight into clinico-mycological profile of Tinea infection in the region.
Conclusion(s)
Dermatophytosis is a common superficial mycotic infection in Saurashtra region where hot and humid climatic condition, poor socio-economic status and compromised hygienic condition play an important role in fungal growth. It mostly affects young males who are working outdoors. Tinea corporis was the most common infection followed by Tinea cruris and the most common isolate being T. rubrum. All the clinically diagnosed tinea infections need to be confirmed by laboratory analysis using both direct microscopy by KOH and culture for proper diagnosis and to know the prevalence of particular dermatophyte.