The current COVID-19 pandemic has not only greatly burdened healthcare system globally but also exposed the medical and paramedical staff to risk of infection. Although the major mode of transmission of this highly infectious disease is via close contact with an infected person i.e., droplet infection due to coughing/sneezing and aerosol generation, few research articles have shown presence of SARS-CoV-2 in blood and serum. This poses a potential risk to health care professionals who are handling these samples. Once the suspected/confirmed case of COVID-19 is admitted in the hospital setting, it requires a battery of clinical chemistry investigations. Laboratory has a vital and indispensable role to play in the management of COVID-19 patients as several biochemical markers are used for prognostication as well as monitoring and guiding treatment in the critical patients. Hence, this evaluation was undertaken to have protocols based on robust recommendations and guidelines to be followed while handling the potentially infective samples in the clinical laboratories in order to ensure safety of the staff. However, these recommendations are based on the limited and rapidly evolving knowledge available at the moment and hence need to be reviewed periodically.
Introduction
In December 2019, few cases of pneumonia like illness were reported at Wuhan, a city in China with a population of more than 11 million. The origin of this illness has been allied to Wuhan wholesale market of sea food [1]. This market also sold live animals such as bats, marmots, snakes, poultry etc. [2]. The infectious zoonotic agent responsible for this disease caused a severe respiratory illness requiring specialised treatment in Intensive Care Unit of hospitals and has since been named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), with the disease named as Coronavirus Disease 2019 (COVID-19) [3]. The modern world with a dynamic population size and high frequency of travel within and outside one’s country has led COVID-19 to spread globally, prompting World Health Organisation (WHO) to declare it a pandemic on 11th March 2020 [4]. According to WHO situation report 148 dated 16th June 2020, the disease has involved all continents, with 79,41,791 confirmed cases in different countries, and 4,34,796 deaths [4].
SARS-CoV-2 had been isolated from a patient in January 2020, and the genome sequencing of this virus has been performed [5]. The genetic sequence of SARS-CoV-2 was officially recognised by WHO in January 2020, and this led to the development of specific Polymerase Chain Reaction (PCR) based diagnostic tests to detect the new infection in various countries [6]. Albeit once admitted in a hospital, an array of clinical and haematological investigations are required besides the confirmatory test to support and guide the management of these cases [7].
Thus, the ongoing pandemic has overwhelmed the healthcare system over the world with humongous workload of critical patients who unfortunately are highly infectious too. A recent research letter from Journal of the American Medical Association (JAMA) showed that COVID-19 was detected in 1% of blood samples received [8], while a study conducted by Zhang W et al., showed that positivity in blood is upto 40% [9] and yet another study by Chang L et al., detected the presence of virus in the blood [10] which makes the people working in clinical chemistry laboratory to be at risk. At face of such unprecedented and unforeseen medical calamity, it is most crucial for health organisations and various international bodies to issue rapid advisory guidelines which would steer the healthcare system in an efficient manner. However, needless to say, there is still paucity of relevant original research articles on experiences and recommendations with regards to clinical chemistry. This may be attributed to sudden surge in cases sparing very little time for analysis and response and also queries generated on regular basis by numerous government and private laboratories for handling of the suspected/confirmed COVID-19 clinical chemistry samples. Hence, this evaluation was undertaken with an aim to provide a perspective of clinical chemistry laboratory in preparation of safe testing of samples of COVID-19 suspected/confirmed cases.
Good clinical laboratory practices: To run a safe clinical chemistry laboratory, the good laboratory practices would includes guidelines for each stage of sample handling, including transport, receipt of biochemical samples from suspected/confirmed COVID-19 cases for analysis. The use of Personnel Protective Equipments (PPE), Disinfectants and Biomedical Waste (BMW) disposal should also be specifically addressing SARS-CoV-2 infection [11-15]. A fool proof safe testing can be achieved by incorporating following recommendations at various stages of handling and disposal of samples from suspected/confirmed COVID-19:
Transport of Biochemical Samples
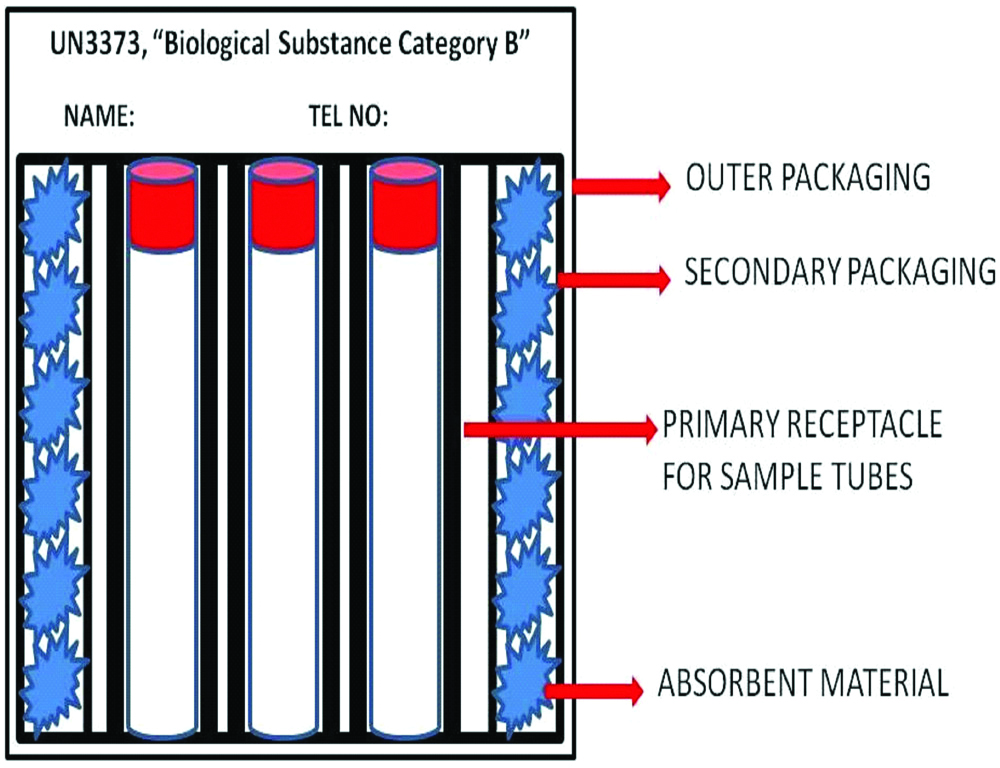
As recommended by Centers for Disease Control and Prevention (CDC), blood and body fluid samples collected from suspected or confirmed cases of COVID-19 should be transported in a special transport box which should have a label marked UN3373, “Biological Substance Category B” [12]. This transport box [Table/Fig-1] is composed of a leak-proof primary container which holds the sample tubes; which is surrounded by a leak-proof secondary packaging and a strong outermost packaging. This box should have minimum dimension of 100×100 mm in at least one of its surfaces. There is an absorbent material which is packed between the primary and secondary container, that would contain the spillage in event of breakage of sample tubes and hence would prevent leakage and contamination of outer surface of the box [16]. Additional wrapping material should be used to segregate individual sample tubes to prevent breakage during transport. Clear and legible labels should be placed on the containers with name and telephone number of the person responsible for shipment.
Schematic representation of sample box for transport of Biochemical Samples from suspected/confirmed COVID-19.
Receipt of Biochemical Samples at Clinical Chemistry Laboratories
Once the sample is received in clinical chemistry laboratory, consider unpacking of the items in the class II Biological Safety Cabinet (BSC) after risk assessment [12,13]. The personnel unpacking and receiving specimens must be adequately trained and should be aware of the hazards involved with the sample. The clinical chemistry laboratories should have secondary containers in which these samples should be placed to minimise the potential of breakage or a spill.
Analysis of Biochemical Samples
The most crucial step is the analysis of the sample in the clinical chemistry laboratory. Centrifugation of the sample has to be performed with utmost care using sealed centrifuge rotors or sample cups which are loaded or unloaded in class II BSC [13]. It is advisable that post-centrifuge one should wait for extra 30 minutes before removing tubes from centrifuge. Also, the vacuum evacuated cap should be held by gauze piece soaked in 1% sodium hypochlorite solution. For the safety of the laboratory technician, the vacuum evacuated cap should be opened with the mouth of the tube away from the technician. During the analysis of the sample, autoanalyser covers should be in place.
Personnel Protective Equipment (PPE) for Personnel Handling Samples
It is advisable to have a rostering of the staff in clinical chemistry laboratory to avoid congregation and maintain physical distance. It is recommended by CDC that the staff working in the clinical biochemistry laboratory handling samples from suspected or confirmed COVID-19 cases must wear gown, scrub suits, disposable gloves and shoe covers. Further they should use the National Institute for Occupational Safety and Health (NIOSH) certified N95 masks, head covers, goggles or face shields [12]. The laboratory personnel should be trained well on correct procedures to don and doff the PPE as well as its disposal after use. There should be area marked in the laboratory for donning and doffing along with a dedicated sink for hand washing [13].
Disinfection of Laboratory
Dry mopping or use of brooms is not recommended as it generates aerosols; instead only dust mop/damp mop are to be used. Daily mopping of floors should be done by use of three bucket method (first with plain water, second with detergent and third with 1% sodium hypochlorite solution) [17]. Possibility of surface contamination by SARS-CoV-2 has to be borne in mind while working in clinical chemistry laboratory. Hence, all the surfaces like tabletops, work platforms, switches, door knobs etc., should be regularly cleaned by using appropriate antimicrobial agents effective against SARS-CoV-2. These include sodium hypochlorite 1,000 ppm (0.1%), 62-71% ethanol, 0.5% hydrogen peroxide, quaternary ammonium compounds, and phenolic compounds [18]. It should be ensured that all the work surfaces used for processing of samples and the autoanalyser must be decontaminated after use. Use of appropriate agent with correct concentration and contact time is the key to efficacy of disinfection that must be followed stringently. The disinfectants must be stored properly as per directions from manufacturers and checked for expiry date before use.
Management of Accidental Spillage of Samples of Suspected/Confirmed COVID-19
While managing a spill in the laboratory, speedy removal of the spillage becomes mandatory. The laboratory should follow stepwise procedure to contain spillage [13]. The personnel managing spillage should wear PPE. The spill should be contained by placing the absorbent material around it and over the spill also. 1.0% sodium hypochlorite solution should be poured on absorbent material over the spillage area and to be kept on it for 15 to 20 minutes. Thereafter, scoop and scraper to be used to collect all material and put in yellow coloured biomedical waste bag. Then, detergent is used to mop the area.
Specific BMW Disposal of Suspected/Confirmed COVID-19 Samples
Standard international guidelines specific to BMW disposal of suspected/confirmed COVID-19 samples are not present. Recent guidelines given by State Pollution Control Boards (SPCBs) are specific to COVID-19 related BMW [19,20]. Accordingly, proper colour coded bags to be maintained in laboratory with proper segregation. A double layered bags/two bags to be used with adequate strength and to avoid leakage. The bins having suspected/confirmed COVID-19 waste should store the waste temporarily with a label “COVID-19” before handing it over to Common Biomedical Waste Treatment Facility (CBWTF). The bags containing suspected/confirmed COVID-19 waste should be labeled as “COVID-19 waste”. The inner and outer surface of the containers/bins/trolleys used for storage of suspected/COVID-19 waste should be disinfected with 1% sodium hypochlorite solution. The laboratory should maintain separate record of COVID-19 waste.
Around the globe, some hospitals have been overburdened with COVID-19 cases. This has led clinical chemistry laboratories to receive large volumes of biochemical samples from the COVID-19 patients who are admitted. The list of common biochemical investigations ordered frequently in COVID-19 are substantial which includes Alanine Aminotransferase, Aspartate aminotransferase, Total bilirubin, Creatinine, Lactate dehydrogenase, Albumin, Cardiac troponin, C reactive protein, Procalcitonin, ferritin, Cytokines (IL-6) [14]. Each of the above parameters requisitioned has a rationale behind it [14].
In a study, involving 205 patients of COVID-19 where qRT-PCR was used to detect SARS-CoV-2 RNA, the blood samples were found to be positive in 1% of cases with mean cycle threshold value of 34.6 indicating viral load of less than 2.6×104 copies/mL while microbiological samples like nasal swab had mean cycle threshold value of 24.3 corresponding to about 1.4×106 copies/mL [8].
Hence, there is an urgent need to implement these guidelines for safe handling of the specimen in a clinical chemistry laboratory. The recommendations can be customised to suit the availability of resources e.g., if the recommended transport containers are not available then transportation boxes can easily be improvised using hard cardboard boxes. Formulating consolidated guidelines custom made for the biochemistry laboratory on basis of roles and resources available would provide concrete directions and clarity on how to handle the samples safely. Also, due to low testing rates and possibility of asymptomatic carriers, we strongly feel that it might be prudent to follow these guidelines universally to bring all the samples being tested in clinical chemistry laboratory under its ambit.
Conclusion(s)
Since the knowledge about COVID-19 is still evolving under scientific studies, it is imperative for clinical chemistry laboratories to keep themselves updated with the latest developments and revise the recommendations as and when required.
[1]. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injurySci China Life Sci 2020 63(3):364-74.10.1007/s11427-020-1643-832048163 [Google Scholar] [CrossRef] [PubMed]
[2]. Lu H, Stratton CW, Tang YW, Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracleJ Med Virol 2019 92(4):401-02.10.1002/jmv.2567831950516 [Google Scholar] [CrossRef] [PubMed]
[3]. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysisTravel Med Infect Dis 2020 34:10162310.1016/j.tmaid.2020.10162332179124 [Google Scholar] [CrossRef] [PubMed]
[4]. World Health Organization. Novel Coronavirus (2019-nCoV) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed: 16 th June 2020 [Google Scholar]
[5]. Torales J, Higgins MO, Castaldelli-Maia JM, Ventriglio A, The outbreak of COVID-19 coronavirus and its impact on global mental healthInternational Journal of Social Psychiatry 2020 66(4):317-20.10.1177/002076402091521232233719 [Google Scholar] [CrossRef] [PubMed]
[6]. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Detection of 2019 novel coronavirus (2019-nCoV) by realtime RT-PCREuro Surveillance 2020 25(3):01-08.10.2807/1560-7917.ES.2020.25.3.2000045 [Google Scholar] [CrossRef]
[7]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaLancet 2020 395:497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[8]. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Detection of SARS-CoV-2 in Different Types of Clinical SpecimensJAMA 2020 323(18):1843-44.10.1001/jama.2020.378632159775 [Google Scholar] [CrossRef] [PubMed]
[9]. Zhang W, Du RH, Li B, Zheng XB, Yang XL, Hu B, Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routesEmerging Microbes & Infections 2020 9(1):386-89.10.1080/22221751.2020.172907132065057 [Google Scholar] [CrossRef] [PubMed]
[10]. Chang L, Zhao L, Gong H, Wang L, Wang L, Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donationsEmerg infect dis 2020 26(7):1631-33./10.3201/eid2607.20083932243255 [Google Scholar] [CrossRef] [PubMed]
[11]. Odega KI, Ehijie I, Ibadin EE, Idomeh FA, Odega DE, Safe laboratory practices in the light of covid-19 pandemic: Way forward in a resource limited settingPreprints 2020 2020:04010310.20944/preprints202004.0103.v1 [Google Scholar] [CrossRef]
[12]. CDC. Interim laboratory biosafety guidelines for handling and processing specimens associated with corona virus disease 2019 (COVID-19). Atlanta: CDC; 2020 [Google Scholar]
[13]. WHO. Laboratory biosafety guidance related to the novel coronavirus (2019-nCoV). Geneva: WHO; 2020 [Google Scholar]
[14]. IFCC. Information guide on COVID-19 (Updated 06 APR 2020). Milan: IFCC; 2020 [Google Scholar]
[15]. Public Health England. Guidance COVID-19: Safe handling and processing for samples in laboratories (Updated 6th Apr 2020). England: Public Health England: 2020 [Google Scholar]
[16]. WHO. Guidance on regulations for the transport of infectious substances. Geneva: WHO; 2007 [Google Scholar]
[17]. National Centre for Disease Control. Guidelines for disinfection of quarantine facility (for COVID-19). New Delhi: NCDC; 202010.46234/ccdcw2020.086 [Google Scholar] [CrossRef]
[18]. United States environmental protection agency. List N: Disinfectants for Use against SARS-CoV-2(Dt 04 Apr 2020). Washington DC: USEPA; 2020 [Google Scholar]
[19]. Central Pollution Control Board. Guidelines for Handling, Treatment, and Disposal of Waste Generated during Treatment/Diagnosis/Quarantine of COVID-19 patients (Mar 2020). New Delhi: CPCB; 2020 [Google Scholar]
[20]. CPCB. Biomedical waste Management & handling rules. New Delhi: Ministry of Environment, Forest and Climate Change, 2016 [Google Scholar]