Introduction
Bay leaf (Syzygium polyanthum) was thought to have potential to treat cardiovascular disease traditionally. Myocardial Infarction (MI) characterised by sudden blockage of coronary artery due to atherosclerosis rupture and following MI, several changes could happen such as expression of Hypoxia-Inducible Factor (HIF) 1α, immune cell infiltration, cytokine release, and fibrosis.
Aim
To study the effect of bay leaf extract on HIF-1α, immune cell infiltration, IL-10 level, and fibrosis in animal model of MI.
Materials and Methods
An Experimental study using animal model of MI was conducted in December 2019. Thirty two Wistar rats were surgically manipulated to have MI. Rats were then divided into two groups: treatment group (with bay leaf extract administration) and control group. Neutrophil and macrophage distribution, collagen (fibrosis) distribution, and HIF-1α expression were examined in infarcted muscle. Additionally, serum was taken to measure IL-10 level. Observation was conducted on day 1, day 4, day 7, day 14 after MI episode. Independent t-test was used to compare serum IL-10 level between groups. Statistical significance was set at p<0.05.
Results
Lower HIF-1α expression was seen in treatment group since day 1, showing better response to ischemia. As for inflammation, decreased neutrophil distribution, increased macrophage distribution was observed in treatment group. Significant increases in IL-10 level were also noticed since day 1 in treatment group (p<0.05 in all observation day). Lower fibrosis was also noticed from day 1 in treatment group. All these effects were further seen in day 4, day 7, and day 14.
Conclusion
Bay leaf extract has potential in reducing ischemia, inflammation, and cardiac fibrosis in animal model of MI.
Coronary disease, Hypoxia, Interleukin 10, Macrophage, Plant extract
Introduction
According to National Health and Nutrition Examination Survey (NHANES), prevalence of cardiovascular disease in United States was 48% in 2013-2016. Of these, 39% were hypertensive, while the remaining 9% had coronary heart disease, heart failure and stroke. Numbers were higher in older age [1]. In Indonesia, Indonesian Basic Health Research (Riskesdas) stated that around 1.5% Indonesian suffered from coronary heart disease. Older age was also the main risk factor [2]. Collaboration has been made in Indonesia to combat noncommunicable disease, yet prevalence and mortality rate of cardiovascular disease, especially heart disease, is still high [3,4].
Despite availability of advance and modern treatment of MI, some people still seek for traditional medicine to treat cardiovascular disease. As Indonesia is rich in natural product, traditional medicine from herbs extract is easily available and widely used along with the modern treatment [5]. Bay leaf (Syzygium polyanthum) is one of traditional herbs that was used to treat several diseases such as cardiovascular disease and diabetes. Recently, studies examining bay leaf, whether about its contents or benefits, are increasing [6,7]. It was found that this leaf contains steroid, phenolic, saponin, flavonoid, and alkaloid [8,9]. Among all substances mentioned, flavonoid is suspected to be benefit to heart. Flavonoid is polyphenol substance which could reduce inflammation, has anti-platelet effect, reduce cholesterol and blood sugar, and also has anti-oxidant activity [10]. Our previous study had confirmed that bay leaf extract could reduce C-reactive protein and myeloperoxidase, thus strengthen anti-inflammatory effect of bay leaf especially in myocardial infartion [11].
MI is one spectrum of acute coronary syndrome, one of the leading cause of death in cardiovascular disease. Atherosclerosis and plaque rupture precede MI and cause coronary artery blockage, as a result, oxygen supply to affected heart muscle is reduced whereas heart has intrinsic oxygen needs that must be maintained to conduct effective contractility [12]. This ischemic state then activate expression of HIF-1 [13]. This protein is transcription factor which function as global regulator of oxygen homeostasis. HIF-1 induces tissue to adapt with hypoxia condition, initiate healing process and with time, restore oxygen supply to tissue. Study on wound healing showed that HIF-1 pathway could help in tissue repair [14].
In early MI, recruitment of immune cell takes place. Neutrophils is one of the earliest cell that infiltrate heart muscle. Proinflammatory cytokine such as IL-6 and TNF-α were also detected [15]. At further stage, macrophage would also be recruited and it would remove necrotic heart cell and apoptotic neutrophil. Several anti-inflamatory cytokine along with angiogenic factor also take place and contribute to cardiac remodelling. All this process need to be well regulated to minimise damage to cardiomyocyte [16].
In this study, effect of bay leaf extract administration was tested against few markers such as HIF-1α, neutrophil and macrophage distribution, collagen (fibrosis) distribution and anti-inflammatory cytokine IL-10 in condition of MI.
Materials and Methods
This is an experimental study using animal model with treatment and control group. Animal handling and its operation procedure was taken place in Animal House, Research Laboratory, School of Medicine, Universitas Brawijaya, Indonesia, while histopathological analysis was conducted in Biochemical and Molecular Biology Laboratory, School of Medicine, Universitas Brawijaya, Indonesia. All procedures were conducted in December 2019. This study was approved by Institutional Ethics Committee of Universitas Sumatera Utara, Indonesia (Reference number 353/TGL/KPEK FK USU-RSUP HAM/2018). Sample size was calculated using Federer’s formula {(t-1) (n-1) ≥15} [17], with the result of 32 samples. Since there was eight groups (t=8) in this study, number of animals in each group was four (n=4).
Animals were 32 white wistar rats provided by Pharmacology Laboratory, School of Medicine, Brawijaya University, Indonesia. Rats were three-month-old, ±300 gram each, and five rats were put in the 40×50 cm cage. To make MI model, all rats underwent surgical procedure [18]. In brief, rats were first anaesthesised using intraperitoneal phenobarbital (40 mg/kg) and premedicated with intramuscular ceftriaxone as prophylaxis. Skin were cut few milimeters below left front leg and thorax were opened through fourth intercostal. Left anterior descending (LAD) artery was ligated and blanching of left ventricular muscle were observed distal to the ligation. Thorax and skin were closed and rats were allowed to recover in its cage. Rats were divided into two groups: treatment group which received bay leaf (Syzygium polyanthum) extract and control group which received no treatment.
Bay leaf extract was suspended in sodium carboxymethyl cellulose 0.5% and administered to treatment group i.e. 3.6 mg/rat (0.72 mL) with nasogastric tube everyday since day 0. Four rats both in control and treatment group were sacrificed at day 1, day 4, day 7, and day 14 after administration of bay leaf extract. Rats were anethesised using ketamine and heart was harvested. Blanching area were seperated and further processed. This sample underwent few steps which comprises of fixation, dehydration, clearing, impregnation, and blocking. After that, sample was cut to 6 μm thickness and stained using haematoxylin and eosin, to observe neutrophil and macrophage distribution, and Masson’s trichrome to observe collagen distribution [19,20]. Immunohistochemistry was conducted to observe HIF-1α expresion by using anti-HIF-1α (SantaCruz Biotechnology, United States). Slides were examined in total of 20 fields by using Nikon E100 microscope with 1000×optical power. Each field must consist of approximately 1500 cells [21]. Picture was documented by using Sony A7 camera at 400×optical power.
Serum IL-10 level was then measured from each rats in all termination day. Blood was first drawn from rats’ lateral vein tail. Blood was left for clotting for about two hours at room temperature and then centrifuged at 2,000×g for 20 minutes. Serum was taken and stored in -20°C until further used. Serum IL-10 level was examined using enzyme-linked immunosorbent assay (ELISA) method (Quantikine® ELISA, Rat IL-10 Immunoassay, R&D System Inc., United States). Procedure was conducted as instructed by manufacturer.
Statistical Analysis
Data analysis was performed using SPSS version 25. Serum IL-10 level in both control and treatment group were tabulised. One-way ANOVA test was used to analyse difference of serum IL-10 level in each group if data was normal. Bonferroni test was used as post-hoc analysis to compare difference each day. Independent t-test was used to compare serum IL-10 level in each termination day between control and treatment group. Statistical significance was set as p<0.05.
Results
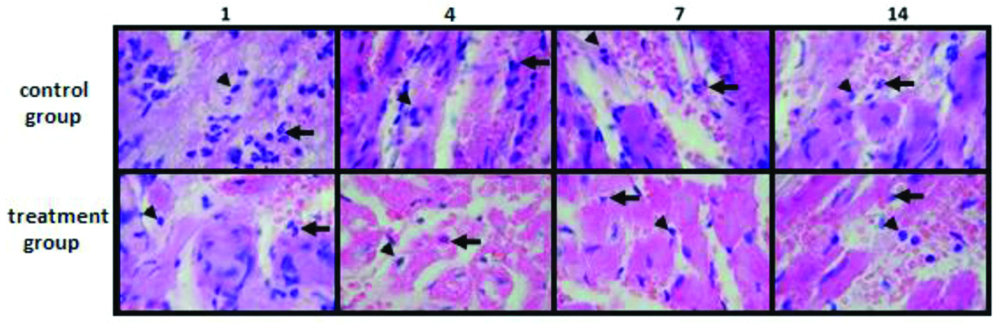
There was difference in the distribution of neutrophil and macrophage in the control group and treatment group. In HE staining, clearly Neutrophils was predominant compared to macrophage in control group as observed in every rats in all termination day. In treatment group, neutrophil distribution was observed in smaller number than control group in the same termination day. Longer treatment reduced neutrofil while increased macrophage distribution [Table/Fig-1]. Furthermore, based on Masson trichrome staining, treatment group had lower amount of collagen (fibrosis) distribution in blanching heart muscle [Table/Fig-2].
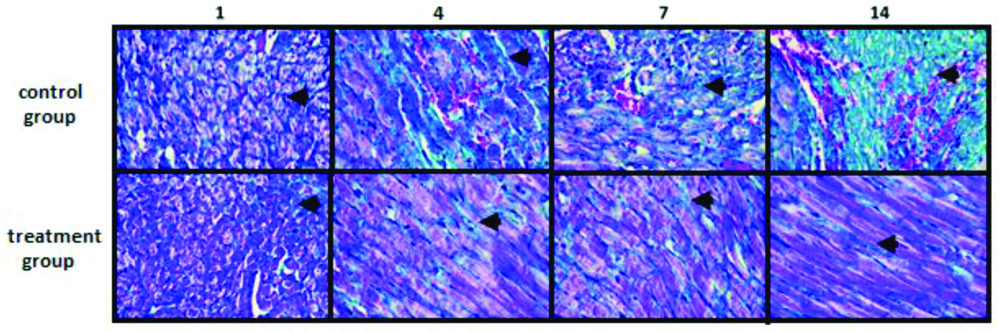
Histopathology of the blanching area of the heart, stained by Masson trichrome. From left to right is histopathology picture on day 1, day 4, day 7, and day 14, respectively. There was reduction in collagen distribution in treatment group compared to control group. Arrow pointed collagen distribution. Magnification: 400× optical power.
Histopathology of the blanching area of the heart, stained by Masson trichrome. From left to right is histopathology picture on day 1, day 4, day 7, and day 14, respectively. There was reduction in collagen distribution in treatment group compared to control group. Arrow pointed collagen distribution. Magnification: 400× optical power.
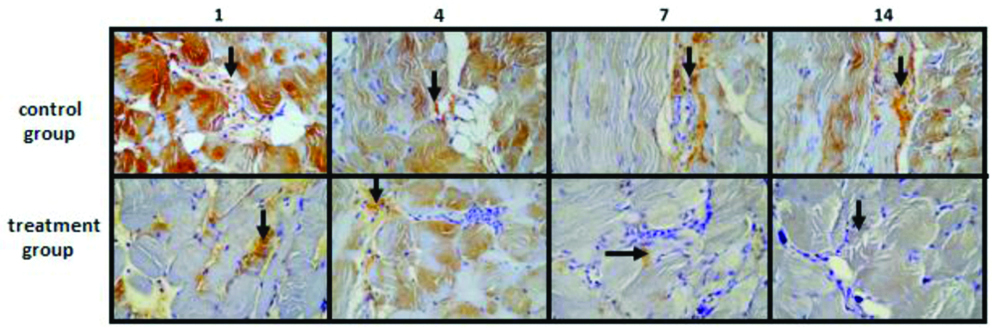
On immunohistochemistry, Expression of HIF-1α was high at day 1 and reduced until day 14 in both groups [Table/Fig-3]. Expression was markedly lower in treatment group compared to control group on day 1. In treatment group, HIF-1α expression was very low in day 14.
Expression of HIF-1α of the blanching area of the heart by using immunohistochemistry. From left to right is expression of HIF-1α on day 1, day 4, day 7, and day 14, respectively. HIF-1α expression was lower in treatment group than control group. Arrow pointed HIF-1α expression. Magnification: 400× optical power.
Assessment of IL-10 level showed that IL-10 level was significantly higher in treatment group, compared to control group in all termination day [Table/Fig-4]. In control group, IL-10 level tend to be higher on late termination day, but it was not statistically significant (p=0.067). On the other hand in treatment group, IL-10 level significantly increase from day 1 until day 14 (p<0.001). In treatment group on post-hoc analysis, IL-10 level was significantly higher from day 7 and day 14 compared to day 1 and day 4 [Table/Fig-4].
IL-10 level in control and treatment group.
| Day | Control group (ng/mL) | Treatment group (ng/mL) | p-value |
|---|
| 1 | 5.00±1.40 | 12.35±2.98 | 0.004 |
| 4 | 7.45±0.57 | 19.32±2.55 | <0.001 |
| 7 | 8.67±2.27 | 31.93±7.65a*,b† | 0.001 |
| 14 | 10.45±4.44 | 33.63±3.93c*,d† | <0.001 |
aday 7 vs day 1, bday 7 vs day 4, cday 14 vs day 1, dday 14 vs day 4 *p<0.001, †p<0.05
control group vs treatment group; independent t-test was used
Difference in each group; ANOVA test with Bonferroni post-hoc analysis was used
Discussion
In this study, LAD artery was ligated to induce myocardial ischemia and infarction showed by blanching of the heart muscle. Ischemia of myocardium induced HIF-1α expression shown in immunohistochemistry in both groups. This expression was abundant one day after LAD artery ligation especially in control group [Table/Fig-3]. This finding is in concordance with Pampin JB et al., and Lee SH et al., which showed that HIF-1α was expressed in early stage of heart ischemia [22,23]. This protein is essential in ischemic state since it could trigger angiogenesis [24]. HIF-1α is one of the first protein expressed in hypoxia and directly induce production of other angiogenic factor such as Vascular Endothelial Growth Factor (VEGF), Delta-Like Ligand 4 (DLL-4), and Platelet Derived Growth Factor beta (PDGF-b) which contribute to the survival of ischemic cells [13,25-27]. Present study showed rats with bay leaf extract had lower HIF-1α expression, which indicated potential of bay leaf extract to resolve heart ischemia. In MI, immune cells orchestration occurs as result of myocardial ischemia. Neutrophil and macrophage are two immune cells that are more extensively studied and together, both cells plays role in inflammation and myocardial remodelling [16,28,29]. In this study, distribution of neutrophil and macrophage was different between rats with bay leaf extract administration and rats in control group. Neutrophil tend to be seen in higher number in control group, while bay leaf extract could reduce neutrophil and increase macrophage distribution in myocardium. Further daily administration of bay leaf extract increase macrophage distribution. Although neutrophil infiltration is also important in early step of cardiac remodelling, it needs to be tightly controlled [30,31]. Increased Polymorphonuclear Leukocyte (PMN) counts were associated with larger infarct size and worse cardiac function [32]. On the other hand, increase macrophage distribution would be benefit for healing after MI [33]. This study showed that bay leaf could have potential in reducing inflammation while inducing better cardiac remodelling.
Interleukin-10 level was also measured as one anti-inflammatory cytokine usually involved in MI. Biswas S et al., and Shrivastava AK et al., stated that IL-10 was lower in patient with myocardial infaction than healthy person, one day after symptom started [15,34]. In present study, bay leaf extract could increase IL-10 level significantly as compared to control group since day 1. Its daily administration, also significantly increased IL-10 level until day 14.
Sziksz E et al., stated that IL-10 was anti-inflammatory cytokine that contribute to cardiac remodelling [35]. In this study, it was found that rats with bay leaf extract administration had lower collagen distribution than control group. This finding is in concordance with current knowledge that IL-10 could induce M2 polarisation, then reduce inflammation and prevent myocardial fibrosis [36].
Classically activated macrophage (M1) and alternatively activated macrophage (M2) usually appeared in different stage of heart ischemia and have their specific role in cardiac remodelling as per few previous studies [16,37].
Limitation(s)
Despite a few benefits of bay leaf extract on MI, exact result still yet to be determined. Isolation of flavonoid or other substance should be conducted before performing similar study. Also, further study should address exact mechanism of its substance in order to provide better knowledge on its effect for ischemia, inflammation and cardiac remodelling. Regarding macrophage recruitment, in this study, polarisation of macrophage was not assessed, whether it was M1 or M2.
Conclusion(s)
Taken together, bay leaf extract or components are potential future treatment of MI. Its mechanisms are possibly by resolving ischemia, reducing inflammation and minimising myocardial fibrosis.
Further study need to be conducted to examine polarisation of macrophage induced by bay leaf extract.
aday 7 vs day 1, bday 7 vs day 4, cday 14 vs day 1, dday 14 vs day 4 *p<0.001, †p<0.05
control group vs treatment group; independent t-test was used
Difference in each group; ANOVA test with Bonferroni post-hoc analysis was used
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 30, 2020
Manual Googling: May 11, 2020
iThenticate Software: Jun 25, 2020 (13%)
[1]. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Heart disease and stroke statistics-2019 update: A report from the American Heart AssociationCirculation 2019 139(10):e56-528. [Google Scholar]
[2]. Ministry of Health of the Republic of Indonesia. The main results of Riskesdas 2018. Ministry of Health of RI, 2018. https://www.kemkes.go.id/resources/download/info-terkini/materi_rakorpop_2018/Hasil%20Riskesdas%202018.pdf. [Accessed 2020 Apr 23] [Google Scholar]
[3]. World Health Organization. WHO country cooperation strategy 2014-2019: Indonesia. New Delhi: WHO; 2016. https://apps.who.int/iris/handle/10665/250550. [Accessed 2020 Apr 23] [Google Scholar]
[4]. Center for Disease Control and Prevention. CDC in Indonesia. CDC, 2020. https://www.cdc.gov/globalhealth/countries/indonesia/pdf/Indonesia_Factsheet.pdf [Google Scholar]
[5]. Elfahmi Woerdenbag HJ, Kayser O, Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological useJ Herbal Med 2014 4(2):51-73.10.1016/j.hermed.2014.01.002 [Google Scholar] [CrossRef]
[6]. Parisa N, Tamzil NS, Harahap DH, Prasasty GD, Hidayat R, Maritska Z, The effect of Leaf Salam Extracts (Syzygium polyanthum) in diabetes mellitus therapy on wistar albino ratsJ Phys Conf Ser 2019 :124610.1088/1742-6596/1246/1/012034 [Google Scholar] [CrossRef]
[7]. Hartanti L, Yonas SMK, Mustamu JJ, Wijaya S, Setiawan HK, Soegianto L, Influence of extraction methods of bay leaves (Syzygium polyanthum) on antioxidant and HMG-CoA Reductase inhibitory activityHeliyon 2019 5(4):e0148510.1016/j.heliyon.2019.e0148531008409 [Google Scholar] [CrossRef] [PubMed]
[8]. Liliwirianis N, Musa NLW, Zain WZWM, Kassim J, Karim SA, Preliminary studies on phytochemical screening of ulam and fruit from MalaysiaE-J Chem 2011 8(S1):S285-88.10.1155/2011/464595 [Google Scholar] [CrossRef]
[9]. Novira PP, Febrina E, Overview of pharmacological activity of bay leaf extract (Syzygium polyanthum (Wight) Walp)Farmaka 2018 16(2):288-97. [Google Scholar]
[10]. Harismah K, Chusniatun Utilization of bay (Eugenia polyantha) leaves as herbal medicines and food flavoringWarta LPM 2016 19(2):110-18.10.23917/warta.v19i2.2742 [Google Scholar] [CrossRef]
[11]. Hasan R, Lindarto D, Siregar GA, Mukhtar Z, The effect of bay leaf extract Syzygiumpolyanthum (Wight) Walp. on C-reactive protein (CRP) and myeloperoxidase (MPO) level in the heart of rat model of myocardial infarctionMed Glas (Zenica) 2020 17(1):41-45. [Google Scholar]
[12]. Frangogiannis NG, Pathophysiology of myocardial infarctionCompr Physiol 2015 5(4):1841-75.10.1002/cphy.c15000626426469 [Google Scholar] [CrossRef] [PubMed]
[13]. Semenza GL, Surviving ischemia: Adaptive responses mediated by hypoxia-inducible factor 1J Clin Inves 2000 106(7):809-12.10.1172/JCI1122311018065 [Google Scholar] [CrossRef] [PubMed]
[14]. Darby IA, Hewitson TD, Hypoxia in tissue repair and fibrosisCell Tissue Res 2016 365(3):553-62.10.1007/s00441-016-2461-327423661 [Google Scholar] [CrossRef] [PubMed]
[15]. Biswas S, Ghoshal PK, Mandal SC, Mandal N, Relation of anti-to pro-inflammatory cytokine ratios with acute myocardial infarctionKorean J Intern Med 2010 25(1):44-50.10.3904/kjim.2010.25.1.4420195402 [Google Scholar] [CrossRef] [PubMed]
[16]. Lambert JM, Lopez EF, Lindsey ML, Macrophage roles following myocardial infarctionInt J Cardiol 2008 130(2):147-58.10.1016/j.ijcard.2008.04.05918656272 [Google Scholar] [CrossRef] [PubMed]
[17]. Badriyah Purwanto The influence of pomelo juice (Citrus maxima var nambangan), vitamin C and lycopene toward MDA level of mouse (Mus musculus) hepatic tissue which exposure by ochratoxinaRes World- J Arts Sci Commer 2016 7(1):74-81.10.18843/rwjasc/v7i1(1)/08 [Google Scholar] [CrossRef]
[18]. Wu Y, Yin X, Wijaya C, Huang MH, McConnell BK, Acute myocardial infarction in ratsJ Vis Exp 2011 48:246410.3791/2464PMC3197402 [Google Scholar] [CrossRef] [PubMed]
[19]. Chang J, Nair V, Luk A, Butany J, Pathology of myocardial infarctionDiagn Histopathol 2013 19(1):07-12.10.1016/j.mpdhp.2012.11.001 [Google Scholar] [CrossRef]
[20]. Bloch W, Korkmaz Y, Classical histological staining procedures in cardiovascular researchIn: Practical Methods in Cardiovascular Research 2005 Berlin, HeidelbergSpringer:485-99.10.1007/3-540-26574-0_24 [Google Scholar] [CrossRef]
[21]. Soini Y, Pääkkö P, Lehto VP, Histopathological evaluation of apoptosis in cancerAm J Pathol 1998 153(4):1041-53.10.1016/S0002-9440(10)65649-0 [Google Scholar] [CrossRef]
[22]. Pampin JB, Rivero SAG, Cepeda XLO, Boquete AV, Vila JF, Fonseca RH, Immunohistochemical expression of HIF-1α in response to early myocardial ischemiaJ Forensic Sci 2006 51(1):120-24.10.1111/j.1556-4029.2005.00014.x16423235 [Google Scholar] [CrossRef] [PubMed]
[23]. Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA, Early expression of angiogenesis factors in acute myocardial ischemia and infarctionN Eng J Med 2000 342(9):626-33.10.1056/NEJM2000030234209041069916226278816 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[24]. Cheng C, Li P, Wang YG, Bi MH, Wu PS, Study on the expression of VEGF and HIF-1α in infarct area of rats with AMIEur Rev Med Pharmacol Sci 2016 20(1):115-19. [Google Scholar]
[25]. Jianqiang P, Ping Z, Xinmin F, Zhenhua Y, Ming Z, Ying G, Expression of hypoxia-inducible factor 1 alpha ameliorate myocardial ischemia in ratBiochem Bioph Res Co 2015 465(4):691-95. [Google Scholar]
[26]. Szylberg Ł, Bodnar M, Michalski J, Maciejewska M, Marszałek A, Inflammation and hypoxia in atherosclerosis, coronary artery disease, and heart failureMed Res J 2015 3(2):46-54. [Google Scholar]
[27]. Parisi Q, Biondi-Zoccai GGL, Abbate A, Santini D, Vasaturo F, Scarpa S, Hypoxia inducible factor-1 expression mediates myocardial response to ischemia late after acute myocardial infarctionInt J Cardiol 2005 99(2):337-39.10.1016/j.ijcard.2003.11.03815749199 [Google Scholar] [CrossRef] [PubMed]
[28]. Hulsmans M, Sam F, Nahrendorf M, Monocyte and macrophage contributions to cardiac remodelingJ Mol Cell Cardiol 2016 93:149-55.10.1016/j.yjmcc.2015.11.01526593722 [Google Scholar] [CrossRef] [PubMed]
[29]. Weinberger T, Schulz C, Myocardial infarction: a critical role of macrophages in cardiac remodelingFront Physiol 2015 6:10710.3389/fphys.2015.0010725904868 [Google Scholar] [CrossRef] [PubMed]
[30]. Ma Y, Yabluchanskiy A, Lindsey ML, Neutrophil roles in left ventricular remodeling following myocardial infarctionFibrogenesis Tissue Repair 2013 6(1):1110.1186/1755-1536-6-1123731794 [Google Scholar] [CrossRef] [PubMed]
[31]. Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotypeEur Heart J 2017 38(3):187-97.10.1093/eurheartj/ehw00228158426 [Google Scholar] [CrossRef] [PubMed]
[32]. Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarctionAm J Cardiol 2009 103(3):333-37.10.1016/j.amjcard.2008.09.08519166685 [Google Scholar] [CrossRef] [PubMed]
[33]. Frantz S, Hofmann U, Fraccarollo D, Schäfer A, Kranepuhl S, Hagedorn I, Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarctionThe FASEB J 2013 27(3):871-81.10.1096/fj.12-21404923159933 [Google Scholar] [CrossRef] [PubMed]
[34]. Shrivastava AK, Singh HV, Raizada A, Singh SK, Serial measurement of lipid profile and inflammatory markers in patients with acute myocardial infarctionEXCLI J 2015 14:517-26. [Google Scholar]
[35]. Sziksz E, Pap D, Lippai R, Béres NJ, Fekete A, Szabó AJ, Fibrosis related inflammatory mediators: Role of the IL-10 cytokine familyMediat Inflamm 2015 2015:76464110.1155/2015/76464126199463 [Google Scholar] [CrossRef] [PubMed]
[36]. Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML, IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activationBasic Res Cardiol 2017 112(3):3310.1007/s00395-017-0622-528439731 [Google Scholar] [CrossRef] [PubMed]
[37]. Weirather J, Hofmann U, Beyersdorf N, Ramos GC, Vogel B, Frey A, Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiationCirc Res 2014 115(1):55-67.10.1161/CIRCRESAHA.115.30389524786398 [Google Scholar] [CrossRef] [PubMed]