Introduction
World Health Organisation (WHO) definition of OPMDs is “clinical presentations that carry a risk of cancer development in the oral cavity, whether in a clinically definable precursor lesion or in clinically normal mucosa” [1]. OSMF was modified to OPMDs by WHO and included in the fourth edition of the World Health Organisation for head and neck tumours [1,2]. Pindborg, in 1966, defined OSMF as “an insidious chronic disease affecting any part of the oral cavity and sometimes pharynx. It is associated with juxta-epithelial inflammatory reaction followed by fibroelastic changes in the lamina propria layer, along with epithelial atrophy which leads to rigidity of the oral mucosa proceeding to trismus and difficulty in mouth opening.” [3]. It is more common in South and East Asia, including India, Pakistan, Bangladesh, southern China and the Pacific Islands. Most patients present with an intolerance to spicy food, rigidity of lip, tongue and palate leading to varying degrees of limitation of opening of the mouth and tongue movement [4]. Various other names has also been given to OSMF by different authors in literature such as idiopathic scleroderma of the mouth, juxta-epithelial fibrosis, idiopathic palatal fibrosis, submucous fibrosis of the palate and pillars, sclerosing stomatitis and diffuse OSMF. The reported prevalence rate of OSMF in India is 0.5% [5], while in Hunan province of China is between 0.1% and 1.9% [6]. OSMF shows a malignant transformation rate as high as 5.2%, and the annual rate of cancer evolution is 0.98% [7]. It appears from current evidence that a combination of various factors could best explain the pathogenesis of OSMF. These include genetic alterations, infectious agents associated with oral cancer including Herpes Simplex Viruses (HSV) and Human Papilloma Virus (HPV) infection, carcinogens such as tobacco and areca nut, nutritional factors including iron depletion and immunologic factors, all of which also have been implicated in the development of oral cancer. The genetic susceptibility to OSMF is due to genomic instability, which is characterised by loss of heterozygosity related to OSMF grading [8]. Genetic factors are the cause of immune abnormalities. It has been reported that the serum levels of IgG, IgA and IgM are increased in OSMF patients. Cys-X-Cys ligand 9 (CXCL9) is an important factor in inducing effector neutrophils and lymphocytes in immune response. It was found that the level of CXCL9 in OSMF increased significantly, indicating that the intensity of immune response increased during the pathogenesis of OSMF [9]. However, it is a well-established fact in many scientific literatures that areca nut is the most important etiologic factor of OSMF.
Areca nut (or betel nut) has been identified as a class carcinogen by the WHO and is the fourth largest addictive substance in the world after tobacco, alcohol and caffeine [10]. About 600 million people around the world chew areca nut. Areca nut chewers are mainly distributed in India, south of China, Pacific Rim islands and Africa, Europe and North America [11], which is basically consistent with the areas with high prevalence of OSMF. The components of areca nut are very complex, and the main alkaloids are arecoline, arecaidine, guvacoline, guvacine, arecaidine-D5,5 and arecaidine-D5,6 [12]. Arecoline has carcinogenicity, mutagenicity and genotoxicity. Compared with those who did not chew betel nut, the risk of OSMF among people who ate areca nut increased by 32 to 109.6 times, with a significant dose-dependent relationship [13]. It is confirmed through the invitro study on the stimulation of human fibroblasts with areca nut extract, arecoline could promote the proliferation of fibroblast resulting in collagen production. Arecoline interferes with the deposition and degradation of Extracellular Matrix (ECM) collagen-related molecules and accelerates local oral mucosal ischemia and hypoxia resulting in changes in local tissue microenvironment, which in turn leads to oral mucosal lamina propria fibrosis.
In this article, various etiological factors behind pathophysiology of OSMF have been reviewed.
A) Increased Collagen Synthesis
Many studies have shown that OSMF is the result of the dynamic imbalance between the synthesis and degradation of ECM. Collagen is the most important component of ECM. Collagen can be secreted by fibroblasts, Endothelial Cells (EC) and some epithelial cells. TGF-β powerfully stimulates the production and deposition of ECM. Arecoline can induce and activate epithelial TGF-β and act on fibroblasts which induce the expression of other fibrogenic cytokines. These changes may lead to excessive ECM deposition of OSMF [14]. TGF-β significantly increases the collagen production by activating procollagen genes such as COL1A2, COL3A1, COL6A1, COL6A3 and COL7A1, simultaneously, it also upregulates the level of procollagen protease and Lysyl Oxidase (LOX) for cross-linkage of collagen fibers. TGF-β also strongly promotes the expression of LOX at mRNA and protein levels [15]. The functional activity of LOX depends on copper. Areca nut has been shown to contain high copper content, which stimulates fibrosis by upregulating the activity of LOX. It has been found that arecoline can upregulate the expression of αvβ6 integrin in Keratinocyte (KC) and promote the activation of TGF-β1 through M4 acetylcholine receptor [16]. In addition, αvβ6-dependent TGF-β1 activation can induce oral fibroblasts to differentiate into myofibroblasts and upregulate genes related to tissue fibrosis [17,18]. On the other hand, Bone Morphogenetic Protein-7 (BMP7) has been shown to reverse TGF-β-mediated collagen expression in mouse models. The role of BMP7 in preventing/reversing fibrosis has been shown to reduce the accumulation of SMAD2 in the nucleus [19]. The up-regulation of fibrogenic TGF-β and the down-regulation of anti-fibrotic BMP7 may be the markers in the pathogenesis of OSMF which disturbed the dynamic balance of ECM production in OSMF.
There is a close relationship between ROS and TGF-β in fibroblasts. Arecoline has been shown to produce ROS through self-oxidation or metabolic activation in cultured cells [20]. When chewing areca nut, ROS may be produced by self-oxidation in saliva or activated by intracellular metabolism [21]. A series of further experiments confirmed that arecoline could induce ROS, within 30 minutes of treatment of HaCaT. Arecoline activates c-Jun N-Terminal Kinase (JNK) through M receptor and ROS, then after JNK phosphorylation, it activates activating transcription factor 2 (ATF2) and TGF-β. These two transcription factors activate the typical TGF-β pathway. It was suggested that arecoline completes the induction and activation of TGF-β2 through the JNK pathway, which contributes to a continuous autocrine cycle of TGF-β and promoted the occurrence of OSMF [22]. Arecoline can also induce human Buccal Mucosa Fibroblasts (BMFs) to synthesise Connective Tissue Growth Factor (CTGF) and Early growth response-1 (Egr-1). CTGF and Egr-1 whose synthesis is mediated by ROS are important mediators for the fibrotic response of OSMF to TGF-β [23,24]. In a study the level of activated TGF-β1 in culture medium was about 2.5 times higher than that in culture medium at 0.2 mmol/l arecoline concentration. The effect of TGF-β1 receptor inhibitor on CTGF was detected. The results showed that TGF-β neutralisation antibody could completely inhibit the synthesis of CTGF and Egr-1 in BMF induced by arecoline. At the same time, different kinds of ROS scavengers were used to stimulate BMFs and it was found that they could completely inhibit the activation of TGF in it. These results suggested that mitochondrial-derived ROS participated in arecoline-induced potential TGF-β1 activation and then induced the synthesis of CTGF and Egr-1 in human buccal fibroblasts and promotes the increase of collagen in the lamina propria of oral mucosa [25,26]. In addition, the expression of Egr-1 was increased when BMF was stimulated by arecoline alone [24]. Because arecoline can lead to excessive increase of middle ROS, the oral mucosa may suffer cellular DNA damage. The damage of cellular DNA caused by different promoters may be one of the mechanisms of OSMF malignant transformation [27].
Transglutaminase-2 (TGM-2), also known as tissue transglutaminase, belongs to the calcium-dependent enzyme family. Thangjam GS et al., confirmed that arecoline in normal gingival fibroblasts, TGM-2 is highly resistant to protease degradation through cross-linking, which leads to the accumulation of ECM and fibrosis [28]. Lee SS et al., confirmed that arecoline can stimulate the production of ROS in human BMF to enhance the expression of TGM-2, suggesting that arecoline-induced TGM-2 expression in human BMF may be partially mediated by ROS. The addition of N-Acetyl-L-Cysteine (NAC) and Epigallocatechin-3 Gallate (EGCG) can inhibit the expression of TGM-2 induced by arecoline, suggesting that NAC and EGCG may play an antioxidant role in the decrease of TGM-2 expression induced by arecoline [29].
Cyclooxygenase (COX) is an endooxidase reductase of prostaglandins (PG), which plays an important role in inflammation and tumourigenesis. When oral fibroblasts were treated with 80 μg/mL arecoline, it was found that the expression of COX-2 was upregulated as early as half an hour, suggesting that this is the early response of cells to arecoline at the transcriptional level. Immunohistochemical detection showed that the expression of COX-2 was increased in moderate fibrosis and disappeared in late fibrosis. This finding is consistent with the histology of the disease because there is no inflammation in the advanced disease [30]. Thrombin is a multifunctional serine protease that promotes platelet aggregation by converting soluble fibrin to insoluble fibrin. It can activate the receptor Proteinase-Activated Receptors (PARs) through specific cell surface receptors called proteases. In addition to the procoagulant effect of PAR-1, PAR-1 has been proved to be the main receptor mediating mitosis, fibrosis, inflammation and ECM remodeling by thrombin [31]. Thrombin exerts its fibrotic effect by inducing CTGF and other secondary mediators [32]. BMFs were treated with arecoline and the expression of PAR-1 in BMFs was observed. It has been mentioned that arecoline can stimulate BMFs to produce CTGF. This suggests that one of the pathogenic mechanisms of OSMF should be that resident cells directly or indirectly trigger the synthesis of PAR-1 expression of CTGF in the response to betel nut stimulation. Further experiments showed that COX-2 inhibitors could inhibit PAR-1 expression stimulated by arecoline, suggesting that COX-2 signal transduction pathway may be involved in PAR-1 expression [33]. Arecoline can activate ROS to stimulate the expression of COX-2 or the production of Prostaglandin E (PGE) in gingival keratinocytes. COX-2-derived PGE-2 is likely to mediate the migration and ECs that drive angiogenesis. The persistent presence of COX-2-derived PGE-2 may stimulate the proliferation of epithelial and ECs, which may contribute to the formation of malignant transformation. Previous studies have shown that COX-2 plays a certain role in the process of local invasion and metastasis of tumour. The increased expression of COX-2 in oral squamous cell carcinoma is related to high recurrence rate, poor radiotherapy response and poor prognosis after treatment [34].
B) Decreased Collagen Degradation
TGF-β regulates collagen degradation by activating Tissue Inhibitor of Metalloproteinase gene (TIMPs) and Plasminogen Activator Inhibitor (PAI) gene. TIMPs is a biological regulator of ECM turnover and a specific inhibitor of matrix Metalloproteinase (MMPs). MMP is composed of a series of structure-related degradation proteases. Their main function is tissue remodeling through the degradation of ECM. The balance between MMPs and TIMPs is disrupted, which may lead to continuous deposition of ECM. When normal fibroblasts and OSMF fibroblasts were cultured with arecoline, OSMF fibroblasts produced more TIMP-1 protein than normal fibroblasts, and TIMP-1 mRNA expression was also higher. Arecoline affects the deposition of ECM by increasing the production of TIMP-1, which is enhanced when fibroblasts are co-cultured with keratinocyte [35]. Another recent study reported that MMP-2 and MMP-9 secreted by oral fibroblasts were rarely found in OSMF. The study further showed that arecoline decreased the secretion of MMP-2 and increased the level of TIMP-1, thus increasing the deposition of collagen in ECM [36]. Plasminogen activator (PLG) is an extracellular proteolytic system that plays an important role in tissue remodeling [37]. Plasmin plays an important role in the activation of pro-MMP, as plasmin promotes the formation of active MMPs, they promote the degradation of collagen. As an inhibitor of PLG, the process of PLG activation is inhibited in OSMF. TGF-β has been shown to stimulate the secretion of PAI-1 in various cell lines and invivo, inhibit existing collagenase and reduce the production of active collagenase, resulting in a significant reduction of collagen degradation and collagen accumulation in OSMF [38]. Maintaining the dynamic balance of connective tissue is very important for the normal function of the tissue. Various cytokines, growth factors and enzymes are considered to play a key role in regulating ECM remodeling. The dynamic balance between MMPs and matrix metalloproteinase TIMPs is one of the decisive factors to maintain the balance and integrity of ECM. With the severity of OSMF histopathology, the expression of MMP-2 increases, which confirms its role in disease progression and malignant trends [39].
C) Hypoxia
Hypoxia has been considered as an important microenvironmental factor in the development of tissue fibrosis. Hypoxia-Inducible Factor-1 (HIF-1) is a kind of α subunit regulated by O2 which is induced by various stimuli is the key mediator of cell adaptation to hypoxia [40]. Hypoxia promotes fibrosis through HIF-1-mediated ECM-modified factors such as PAI-1 [41]. No matter at the mRNA level or at the protein level, arecoline significantly increased the expression of PAI-1mRNA and protein in BMFs. The results showed that HIF-1α inhibitor could significantly inhibit the expression of PAI-1 protein under anoxic condition, and arecoline could upregulate the expression of PAI-1 protein under anoxic condition than that under normoxic condition. Hypoxia through HIF-1α may promote the formation of fibrosis by stimulating PAI-1 to promote the accumulation of ECM in oral submucosa. It is suggested that HIF-1α may play its role in promoting fibrosis by upregulating the accumulation of PAI-1 protein induced by arecoline, and there is local hypoxia in OSMF tissue [42]. Tissue fibrosis in OSMF may lead to local hypoxia by blocking blood vessels, thus stimulating the upregulation of HIF-1α. In addition, the degree of cell dysplasia is related to the expression of HIF-1α, suggesting that tissue hypoxia plays an important role in the malignant transformation of fibrosis [8].
D) Micro Vessel
Arecoline toxicity may lead to the decrease of blood vessels in OSMF. With the progress of fibrosis, mucosal blood vessels decrease, and microvascular changes are the adaptive response of mucous membrane to hypoxia induced by progressive fibrosis [43]. Arecoline has cytotoxic and growth inhibitory effect on vascular ECs which is caused by cell cycle arrest and apoptosis. Long-term exposure to low concentration of arecoline is also toxic to EC [44]. EC is the basic structure of microvessels the target cell and effector in the pathological process of the occurrence and development of OSMF. The cytokines secreted after EC injury can also stimulate the proliferation of smooth muscle cells, thicken the vascular wall, narrow the lumen, tissue ischemia and hypoxia, insufficient oxygen supply of blood perfusion, and aggravate tissue fibrosis. In one of the study, immunohistochemistry showed that the expression of Basic Fibroblast Growth Factor (BFGF) and Platelet-Derived Growth Factor (PDGF) increased in the tissues affected by OSMF [45]. Low concentration of arecoline had obvious cytotoxic effect on EC and cell proliferation was significantly inhibited. It has been confirmed that arecoline possessed cytotoxic and growth inhibitory effect on vascular ECs. Due to the cell cycle arrest and apoptosis of EC induced by arecoline, long-term exposure to low concentration of arecoline is also toxic to vascular ECs. BFGF has strong proliferative activity on fibroblasts and ECs, because of lack of conventional secretory signal sequence, then which is considered playing an important role in the occurrence and development of many fibrotic diseases including OSMF [46]. Data shows that arecoline-induced vascular BFGF combines with endothelial necrosis by increasing the expression of growth factor, thus promoting fibroblast proliferation and the occurrence of OSMF [47]. Studies have shown that arecoline can stimulate ECs to secrete Nitric Oxide (NO), to dilate blood vessels. NO in ECs is produced by L-arginine as substrate under the action of Nitric Oxide Synthase (NOS). NO is an important vasodilator, which leads to vasodilation due to the decrease of free Ca2+ concentration. Arecoline inhibits the damage of vascular endothelial function induced by high glucose. The mechanism may be related to arecoline activating M receptor on ECs, increasing the production of NO and arecoline reducing the production of lipid peroxides and inhibiting oxidative stress [48].
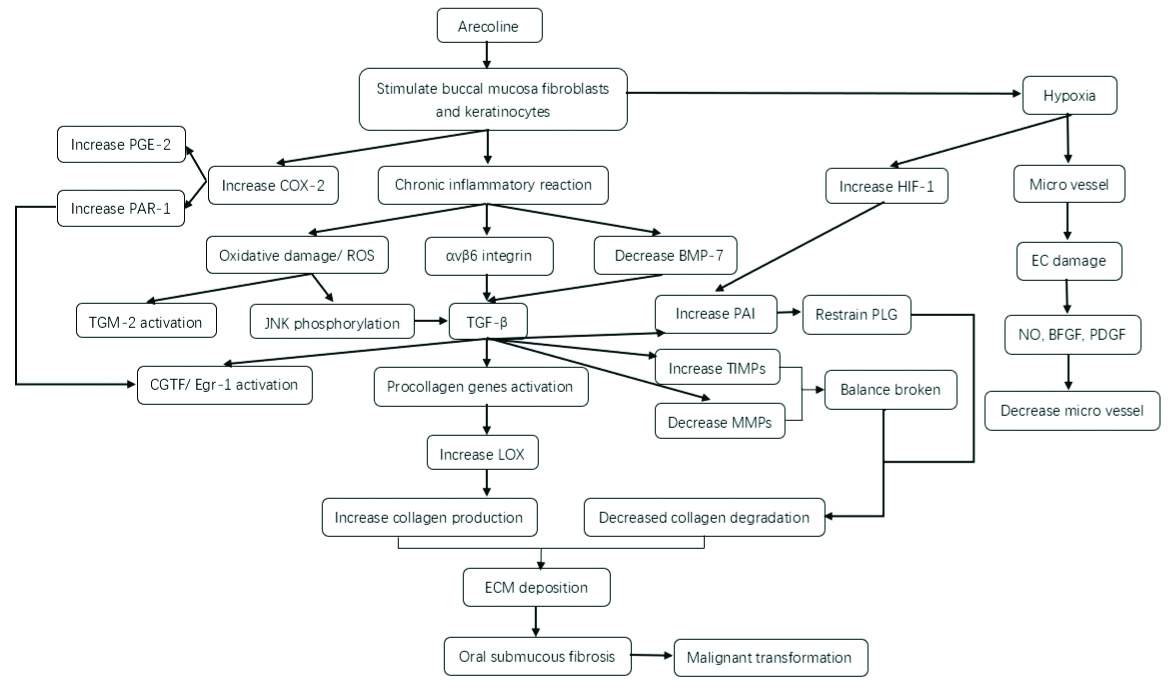
As one of the main pathogenic factors of OSMF, arecoline affects the changes of molecular level in the pathways related to the increase of collagen production, the decrease of collagen degradation and hypoxia through long-term chronic stimulation of oral mucosa related lesions of OSF. The changes of molecular mechanism of arecoline in OSMF and the relationship between them are shown in [Table/Fig-1].
Molecular Mechanism Associated with Arecoline of Oral Submucous Fibrosis.
Arecoline can stimulate buccal mucosa fibroblasts and oral keratinocytes, cause chronic inflammation and hypoxia reaction. Chronic inflammation can induce ROS, αvβ6 and BMP7 to activate Transforming Growth Factor-β (TGF-β), which leads to the change of fibrosis related factors and increase collagen production. At the same time, TGF-β can also destroy the balance between TIMP and MMP, which reduce collagen degradation. Oral mucosal hypoxia can lead to microvascular changes in oral mucosa; These change leads to excessive deposition of ECM and occurrence of OSF in oral mucosa
Molecular-Based Therapy
In view of the important role of TGF-β in OSMF and its malignant transformation, different components of TGF-β signaling pathway provide a potential attractive therapeutic target for the treatment of OSMF. Targeting TGF-β and its receptors and following the downstream steps of its signal pathway, a new therapy for OSMF may be found. Glabridin is extracted from licorice root. It is a kind of isoflavone or natural phenolic compound with antioxidant and anti-inflammatory properties. It can inhibit the production of TGF-β in human buccal fibroblasts [49].
Epigallocatechin-3-Gallate (EGCG) is the most abundant catechin in tea. It is an antioxidant that inhibits intracellular ROS. Invitro studies have shown that EGCG inhibits several fibrogenic genes, such as Egr-1, CTGF-2 and TGM-2. EGCG completely blocks arecoline-induced Egr-1 and gel contraction of bone marrow stromal cells [50].
Hyperbaric Oxygen Therapy (HBOT) increases the oxygen tension and transport of hypoxic tissue and can be used as an adjuvant therapy for fibrosis involving hypoxia. HBOT inhibits the activity of fibroblasts and has the properties of anti-inflammation and antioxidation, so it has the therapeutic effect of OSMF [51]. Tea pigment can reduce high blood viscosity, improve microcirculation and relieve local ischemia. Tea pigment can significantly increase the degree of mouth opening in patients with OSMF with hyper viscosity. The efficacy of peripheral vasodilator bufenin hydrochloride is similar to that of tea pigment. Experimental studies have found that bufenin hydrochloride can quickly improve the subjective symptoms of patients with OSMF [52].
Conclusion(s)
The existing literature shows that the main pathogenic factor of OSMF is arecoline the main component of areca nut. By interfering with the molecular process of deposition and degradation of ECM, the main related upstream and downstream factors of TGF-β can be affected, resulting in the imbalance between collagen synthesis and degradation in the lamina propria of oral mucosa which can induce OSMF and its malignant transformation. Through strengthening clinical and basic research in the future, the relationship between molecules as a marker and targeted drug for the diagnosis of the disease can be explored, to provide ideas for OSMF treatment.
[1]. Reibel J, Gale N, Hille J, Hunt JL, Lingen M, Muller S, Oral potentially malignant disorders and oral epithelial dysphasia. In: El-Nagger AK, Chan JKC, Grandis JR, Takata T, Slootweg PPJ, editorsWHO Classification of Head and Neck Tumours 2017 20174th edLyon, FranceIARC:4 [Google Scholar]
[2]. Warnakulasuriya S, Johnson NW, Van Der Waal I, Nomenclature and classification of potentially malignant disorders of the oral mucosaJ Oral Pathol Med 2007 36(10):575-80.10.1111/j.1600-0714.2007.00582.x17944749 [Google Scholar] [CrossRef] [PubMed]
[3]. Pindborg JJ, Oral submucous fibrosis as a precancerous conditionJ Dent Res 1966 45:546-50.10.1177/00220345660450031701 [Google Scholar] [CrossRef]
[4]. Khan S, Chatra L, Prashanth SK, Veena KM, Rao PK, Pathogenesis of oral submucous fibrosisJ Cancer Res Ther 2012 8(2):199-203.10.4103/0973-1482.9897022842361 [Google Scholar] [CrossRef] [PubMed]
[5]. Aziz SR, Oral submucous fibrosis: Case report and review of diagnosis and treatmentJ Oral Maxil Surg (02782391) 2008 66(11):2386-89.10.1016/j.joms.2008.06.06418940512 [Google Scholar] [CrossRef] [PubMed]
[6]. Zhang SS, Li WH, Gao YJ, Liu ZW, Liu L, Tang JQ, Betel-quid and oral submucous fibrosis: A cross-sectional study in Hunan province, ChinaJ Oral Pathol Med 2012 41(10):748-54.10.1111/j.1600-0714.2012.01166.x22607258 [Google Scholar] [CrossRef] [PubMed]
[7]. Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtypeHead Neck 2020 42(3):539-55.10.1002/hed.2600631803979 [Google Scholar] [CrossRef] [PubMed]
[8]. Ekanayaka RP, Tilakaratne WM, Oral submucous fibrosis: Review on mechanisms of malignant transformationOr Surg Or Med Or Pa 2016 122(2):192-99.10.1016/j.oooo.2015.12.01827289264 [Google Scholar] [CrossRef] [PubMed]
[9]. Li N, Hu Q, Jiang C, Guo F, Munnee K, Jian X, Cys-X-Cys ligand 9 might be an immunological factor in the pathogenesis of oral submucous fibrosis and its concomitant oral lichenoid lesionClin Oral Invest 2013 17(4):1251-58.10.1007/s00784-012-0799-922821431 [Google Scholar] [CrossRef] [PubMed]
[10]. Sullivan RJ, Hagen EH, Psychotropic substance-seeking: Evolutionary pathology or adaptation?Addiction 2002 97(4):389-400.10.1046/j.1360-0443.2002.00024.x11964056 [Google Scholar] [CrossRef] [PubMed]
[11]. Lee CH, Ko AMS, Warnakulasuriya S, Yin BL, Sunarjo Zain RB, Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: An international collaborative study by Asian betel-quid consortium of south and east AsiaInt J Cancer 2011 129(7):1741-51.10.1002/ijc.2580921128235 [Google Scholar] [CrossRef] [PubMed]
[12]. Jain V, Garg A, Parascandola M, Chaturvedi P, Khariwala SS, Stepanov I, Analysis of Alkaloids in Areca Nut-Containing Products by Liquid Chromatography-Tandem Mass SpectrometryJ Agric Food Chem 2017 65(9):1977-83.10.1021/acs.jafc.6b0514028190359 [Google Scholar] [CrossRef] [PubMed]
[13]. Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S, Oral submucous fibrosis: Review on aetiology and pathogenesisOral Oncol 2006 42(6):561-68.10.1016/j.oraloncology.2005.08.00516311067 [Google Scholar] [CrossRef] [PubMed]
[14]. Pant I, Kumar N, Khan I, Rao SG, Kondaiah P, Role of areca nut induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of oral submucous fibrosisPLoS ONE 2015 10(6):01-19.10.1371/journal.pone.012925226107172 [Google Scholar] [CrossRef] [PubMed]
[15]. Arakeri G, Rai KK, Hunasgi S, Merkx MAW, Gao S, Brennan PA, Oral submucous fibrosis: An update on current theories of pathogenesisJ Oral Pathol Med 2017 46(6):406-12.10.1111/jop.1258128391620 [Google Scholar] [CrossRef] [PubMed]
[16]. Moutasim KA, Jenei V, Sapienza K, Marsh D, Weinreb PH, Violette SM, Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosisJ Pathol 2011 223(3):366-77.10.1002/path.278621171082 [Google Scholar] [CrossRef] [PubMed]
[17]. Khan I, Agarwal P, Thangjam GS, Radhesh R, Rao SG, Kondaiah P, Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosisGrowth Factors (Chur, Switzerland) 2011 29(4):119-27.10.3109/08977194.2011.58283921591998 [Google Scholar] [CrossRef] [PubMed]
[18]. Khan I, Kumar N, Pant I, Narra S, Kondaiah P, Activation of TGF-β pathway by areca nut constituents: A possible cause of oral submucous fibrosisPlos One 2012 7(12):e51806-e.10.1371/journal.pone.005180623284772 [Google Scholar] [CrossRef] [PubMed]
[19]. Izumi N, Mizuguchi S, Inagaki Y, Saika S, Kawada N, Nakajima Y, BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2Am J Physiol-lung C 2006 290(1):L120-L6.10.1152/ajplung.00171.200516126788 [Google Scholar] [CrossRef] [PubMed]
[20]. Chang MC, Ho YS, Lee PH, Chan CP, Lee JJ, Hahn LJ, Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: Association of glutathione, reactive oxygen species and mitochondrial membrane potentialCarcinogenesis 2001 22(9):1527-35.10.1093/carcin/22.9.152711532876 [Google Scholar] [CrossRef] [PubMed]
[21]. Illeperuma RP, Kim DK, Park YJ, Son HK, Kim JY, Kim J, Areca nut exposure increases secretion of tumour-promoting cytokines in gingival fibroblasts that trigger DNA damage in oral keratinocytesInt J Cancer 2015 137(11):2545-57.10.1002/ijc.2963626076896 [Google Scholar] [CrossRef] [PubMed]
[22]. Pant I, Rao SG, Kondaiah P, Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-beta pathway in precancerous Oral Submucous FibrosisSci Rep 2016 6:3431410.1038/srep3431427708346 [Google Scholar] [CrossRef] [PubMed]
[23]. Deng YT, Chen HM, Cheng SJ, Chiang CP, Kuo MY, Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcuminOral Oncol 2009 45(9):e99-e105.10.1016/j.oraloncology.2009.04.00419457704 [Google Scholar] [CrossRef] [PubMed]
[24]. Hsieh YP, Chen HM, Chang JZ, Chiang CP, Deng YT, Kuo MY, Arecoline stimulated early growth response-1 production in human buccal fibroblasts: Suppression by epigallocatechin-3-gallateHead Neck 2015 37(4):493-97.10.1002/hed.2361424436257 [Google Scholar] [CrossRef] [PubMed]
[25]. Chang JZC, Yang W-H, Deng YT, Chen HM, Kuo MYP, EGCG blocks TGFβ1-induced CTGF by suppressing JNK and p38 in buccal fibroblastsClin Oral Invest 2013 17(2):455-61.10.1007/s00784-012-0713-522415218 [Google Scholar] [CrossRef] [PubMed]
[26]. Hsieh YP, Wu KJ, Chen HM, Deng YT, Arecoline activates latent transforming growth factor beta1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallateJ Formos Med Assoc 2018 117(6):527-34.10.1016/j.jfma.2017.07.00328720506 [Google Scholar] [CrossRef] [PubMed]
[27]. Hu CW, Chao MR, Direct-acting DNA alkylating agents present in aqueous extracts of areca nut and its productsChem Res Toxicol 2012 25(11):2386-92.10.1021/tx300252r23033867 [Google Scholar] [CrossRef] [PubMed]
[28]. Thangjam GS, Agarwal P, Khan I, Verma UP, Balapure AK, Rao SG, Transglutaminase-2 regulation by arecoline in gingival fibroblastsJ Dent Res 2009 88(2):170-75.10.1177/002203450832963319278990 [Google Scholar] [CrossRef] [PubMed]
[29]. Lee SS, Chen YJ, Tsai CH, Huang FM, Chang YC, Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generationClin Oral Investig 2016 20(5):1029-34.10.1007/s00784-015-1579-026336810 [Google Scholar] [CrossRef] [PubMed]
[30]. Tsai CH, Chou MY, Chang YC, The up-regulation of cyclooxygenase-2 expression in human buccal mucosal fibroblasts by arecoline: A possible role in the pathogenesis of oral submucous fibrosisJ Oral Pathol Med 2003 32(3):146-53.10.1034/j.1600-0714.2003.00004.x12581384 [Google Scholar] [CrossRef] [PubMed]
[31]. Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosisAm J Pathol 2005 166(5):1353-65.10.1016/S0002-9440(10)62354-1 [Google Scholar] [CrossRef]
[32]. Chambers RC, Laurent GJ, Coagulation cascade proteases and tissue fibrosisBiochem Soc Trans 2002 30(2):194-200.10.1042/bst0300194 [Google Scholar] [CrossRef]
[33]. Tsai CH, Lee SS, Huang FM, Chang YC, Regulation of protease-activated receptor-1 expression in human buccal fibroblasts stimulated with arecolineHead Neck 2013 35(9):1314-18.10.1002/hed.2313022965839 [Google Scholar] [CrossRef] [PubMed]
[34]. Lalier L, Pedelaborde F, Braud C, Menanteau J, Vallette FM, Olivier C, Increase in intracellular PGE2 induces apoptosis in Bax-expressing colon cancer cellBMC Cancer 2011 11:15310.1186/1471-2407-11-15321524287 [Google Scholar] [CrossRef] [PubMed]
[35]. Xia L, Tian-You L, Yi-Jun G, Dong-Sheng T, Wen-Hui L, Arecoline and oral keratinocytes may affect the collagen metabolism of fibroblastsJournal of Oral Pathology & Medicine: Official Publication of The International Association of Oral Pathologists and The American Academy of Oral Pathology 2009 38(5):422-26.10.1111/j.1600-0714.2009.00758.x19320801 [Google Scholar] [CrossRef] [PubMed]
[36]. Chang YC, Yang SF, Tai KW, Chou MY, Hsieh YS, Increased tissue inhibitor of metalloproteinase-1 expression and inhibition of gelatinase A activity in buccal mucosal fibroblasts by arecoline as possible mechanisms for oral submucous fibrosisOral Oncol 2002 38(2):195-200.10.1016/S1368-8375(01)00045-8 [Google Scholar] [CrossRef]
[37]. Andreasen PA, Egelund R, Petersen HH, The plasminogen activation system in tumour growth, invasion, and metastasisCell Mol Life Sci 2000 57(1):25-40.10.1007/s00018005049710949579 [Google Scholar] [CrossRef] [PubMed]
[38]. Yang SF, Hsieh YS, Tsai CH, Chou MY, Chang YC, The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosisOral Oncol 2003 39(4):367-72.10.1016/S1368-8375(02)00123-9 [Google Scholar] [CrossRef]
[39]. Shrestha A, Carnelio S, Evaluation of matrix metalloproteinases-2 (MMP-2) and tissue inhibitors of metalloproteinases-2 (TIMP-2) in oral submucous fibrosis and their correlation with disease severityKathmandu Univ Med J (KUMJ) 2013 11(44):274-81.10.3126/kumj.v11i4.1252124899319 [Google Scholar] [CrossRef] [PubMed]
[40]. Schofield CJ, Ratcliffe PJ, Oxygen sensing by HIF hydroxylasesNat Rev Mol Cell Biol 2004 5(5):343-54.10.1038/nrm136615122348 [Google Scholar] [CrossRef] [PubMed]
[41]. Li X, Kimura H, Hirota K, Kasuno K, Torii K, Okada T, Synergistic effect of hypoxia and TNF-β on production of PAI-1 in human proximal renal tubular cellsKidney International 2005 68(2):569-83.10.1111/j.1523-1755.2005.00435.x16014034 [Google Scholar] [CrossRef] [PubMed]
[42]. Tsai CH, Lee SS, Chang YC, Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecolineJ Oral Pathol Med 2015 44(9):669-73.10.1111/jop.1228425367145 [Google Scholar] [CrossRef] [PubMed]
[43]. Fang CY, Han WN, Fong DY, [A morphometric study on the microvessel in oral submucous fibrosis]Hunan yi ke da xue xue bao=Hunan yike daxue xuebao=Bulletin of Hunan Medical University 2000 25(1):55-57. [Google Scholar]
[44]. Tseng SK, Chang MC, Su CY, Chi LY, Chang JZC, Tseng WY, Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cellsClin Oral Invest 2012 16(4):1267-73.10.1007/s00784-011-0604-121847594 [Google Scholar] [CrossRef] [PubMed]
[45]. Bishen KA, Radhakrishnan R, Satyamoorthy K, The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesisJ Oral Pathol Med 2008 37(7):402-11.10.1111/j.1600-0714.2008.00649.x18298475 [Google Scholar] [CrossRef] [PubMed]
[46]. Makino T, Jinnin M, Muchemwa FC, Fukushima S, Kogushi-Nishi H, Moriya C, Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK1/2 and JNK pathwaysBr J Dermatol 2010 162(4):717-23.10.1111/j.1365-2133.2009.09581.x19995368 [Google Scholar] [CrossRef] [PubMed]
[47]. Ullah M, Cox S, Kelly E, Moore MA, Zoellner H, Arecoline increases basic fibroblast growth factor but reduces expression of IL-1, IL-6, G-CSF and GM-CSF in human umbilical vein endotheliumJ Oral Pathol Med 2015 44(8):591-601.10.1111/jop.1227625529330 [Google Scholar] [CrossRef] [PubMed]
[48]. Kuo F, Wu D, Yuan SF, Hsiao K, Wang Y, Yang Y, Effects of arecoline in relaxing human umbilical vessels and inhibiting endothelial cell growthJ Perinat Med 2005 33(5):399-405.10.1515/JPM.2005.07216238534 [Google Scholar] [CrossRef] [PubMed]
[49]. Lee PH, Chu PM, Hsieh PL, Yang HW, Chueh PJ, Huang YF, Glabridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGFβ/smad signalingEnviron Toxicol 2018 33(2):248-55.10.1002/tox.2251229119715 [Google Scholar] [CrossRef] [PubMed]
[50]. Yu-Ping H, King-Jean W, Hsin-Ming C, Yi-Ting D, Hsieh YP, Wu KJ, Arecoline activates latent transforming growth factor β1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallateJ Formos Med Assoc 2018 117(6):527-34.10.1016/j.jfma.2017.07.00328720506 [Google Scholar] [CrossRef] [PubMed]
[51]. Ye X, Zhang J, Lu R, Zhou G, HBO: A possible supplementary therapy for oral potentially malignant disordersMedical Hypotheses 2014 83(2):131-36.10.1016/j.mehy.2014.05.01124908359 [Google Scholar] [CrossRef] [PubMed]
[52]. Sharma JK, Gupta AK, Mukhija RD, Nigam P, Clinical experience with the use of peripheral vasodilator in oral disordersInt J Oral Max Surg 1987 16(6):695-99.10.1016/S0901-5027(87)80055-3 [Google Scholar] [CrossRef]