Smoking is a major cause for many preventable diseases worldwide. Smoking has been linked to many health problems such as cardiovascular diseases, cancers, periodontitis and colour change of teeth [1-3]. Recently, the consumption of tobacco has witnessed a significant change since the introduction the ECs, also described as Electronic Nicotine Delivery Systems (ENDS) [4]. The use of ECs which is a non-cigarette tobacco product, has increased over the last six years as a way of quitting smoking or as an alternative to tobacco cigarettes [5].
The basic structure of ECs includes a heating element, a battery as a power source, a mouthpiece used for inhalation and a reservoir or cartridge that is filled with the liquid juice called electronic juice (e-juice) which contains the nicotine [4]. The inhaled aerosol is generated by heating the e-juice which is mainly made of glycerol, propylene glycol, nicotine and flavourings, while tobacco cigarettes are composed of many particulates and more than 4000 chemicals that have been linked to direct or indirect harm to human health [4,6].
The market has different brands of ECs and variable flavours of e-juice. Some reports on commercial ECs showed lower level of toxic substances [1,7]. On the other hand, other reports suggested that the effect of ECs on physiological system is as dangerous as (or more dangerous than) traditional smoking [8-10].
Due to a paucity of existing literature on the widespread and unregulated use of ECs, more studies are required to investigate the different effects of ECs on human health. The oral and nasal areas harbour variable commensal microorganisms such as Streptococcus viridans, Staphylococcus aureus and Candida spp [11-13]. Disruption of the flora in these areas can precipitate to colonisation of pathogenic types such as MRSA and Haemophilus influenza leading local and systemic diseases such as gingivitis, pneumonia and bacteremia [14], therefore it is important to study if host commensals might be affected by ECs in nasal and oral areas.
The aim of the current pilot study was to examine the microbial profile and its antibiotic susceptibility in the oral and nasal cavity of ECs users in comparison to smokers and non-smokers, and to check for contamination in e-juices used by the participants.
Materials and Methods
Study Design and Samples
This pilot study was conducted at the Faculty of Medicine in the University of Mu’tah, Jordan in the period from July 2019 to October 2019. Approval for the study was obtained from the scientific and the ethics committee at the faculty of medicine, University of Mutah, Jordan, approval number 201930.
A total of 30 non-smokers (who never smoked before), 30 current tobacco smokers (15-20 cigarettes per day) and 30 ECs user (who were previous tobacco smokers and using EC for more than six months) were enrolled for the study. Non-smokers were considered as controls. Classification criteria of study groups as described by Stewart J et al., was followed [15]. The sample size of this pilot study was calculated as previously described by Browne RH [16].
All participants were university students, in their first to third year of study, aged 19-21 years. A written informed consent was obtained from all participants.
Inclusion criteria for all participants were age more than 18 years and ECs users (at least six months). Total duration of using ECs was 6-8 months).
Exclusion criteria were recent (last three months) antibiotic use, recent hospital admission, previous positive history of MRSA colonisation or infection and current mouth or Upper Respiratory Tract Infection (URTI) or ulcers such as pharyngitis, gingivitis and herpetic ulcers.
Data Collection and Sampling
Data was collected using a structured questionnaire that was filled and signed by all participants. Questionnaire included baseline data to assess some relevant risk factors for the MRSA carriage and to classify smoking status and history of the ECs users participants, in order to check inclusion or exclusion criteria of the study. The data filled by participants included information such as age, sex, smoking and ECs history (duration, amount, type of juice and nicotine concentration), recent (last three months) hospital admission, and recent antibiotic intake, current mouth or Respiratory Tract Infections (RTI). For EC users, questions were also asked whether they have noticed any decrease in respiratory tract symptoms (bronchitis) and early morning phlegm production since they started using ECs. Each EC user was asked to bring their own e-juice to the sampling area for culturing.
The questionnaire was pretested by different experts (microbiologists and epidemiologist) and then pilot testing was done on 30 students. This data was not included in the analysis. Sampling for each participant was performed by rotating two sterile cotton swabs one in the vestibule of both anterior nares and one from buccal mucosa (cheek) and adjacent tongue as previously described by Lo WT et al., [17].
Sample Analysis and Antibiotic Sensitivity
The majority of the collected samples were processed immediately by inviting participants to attend the microbiology laboratory with the e-juice used by each one. If a delay in the processing of samples was expected, they were collected and stored in transport medium (Venturi Transystem, Copan Diagnostics, Corona, CA, USA) that was kept in an ice box and transported within less than one hour at a temperature between 4-8°C to the Microbiology Laboratory.
Nasal swabs were spread on selected agars including blood agar and mannitol salt agar. Swabs from buccal mucosa and tongue were inoculated on hypertonic Sabouraud Dextrose Agar (SDA) for Candida spp identification. For Streptococcus viridians isolation, oral swabs were inoculated on Trypticase Soy-Sucrose-Bacitracin (TYS20B) overnight and the catalase negative colonies were identified next day and inoculated on blood agar for further identification as described by Abu-zineh R et al., [18].
Electronic-liquid juice samples from EC user participants were also cultured to detect for any microbial growth. The e-juice was processed as a body fluid sample using the standard aerobic culture methods for processing clinical specimens [19-21]. One mL of e-juice from each participant was directly taken from the bottle of the juice using a sterile Pasteur pipette and inoculated into brain heart infusion and then incubated for 4 hours at 35-37°C. The broth was then subcultured on blood agar, mannitol salt agar, SDA and EMB agar (Eosin Methylene Blue) and incubated 35-37°C. The culture was then examined for any growth 24-48 hours later.
Plates were incubated as appropriate at 35-37°C (Streptococcus viridians incubated with 5-10% CO2) and examined for growth. Catalase negative colonies from TYS20B were selected and sub-cultured on blood agar with 5 μg optochin disc for another 24 hours. The growing Staphylococcus spp. colonies were identified by typical colony morphology, Gram’s staining, anaerobic utilisation of glucose and mannitol, and positive catalase production and tube coagulase test.
On the other hand, presumed Streptococcus viridians colonies were identified as described by Abu-zineh R et al., [18]. Briefly, optochin resistance colonies were selected and cultured on bile esculin and 6.5% NACL to exclude Enterococcus spp. Gram positive cocci that were catalase and bile esculin negative, optochin resistance and did not grow in 6.5% NACL were ultimately identified as Streptococcus viridians. Presumptive Candida albicans were identified after 24-48 hours incubation by colony morphology, gram stain and germ tube test as previously described [22,23].
Screening for methicillin resistance was performed using 30 μg/mL cefoxitin in Mueller-Hinton agar according to CLSI guidelines [24]. Antibiotic sensitivity was performed using Kirby Bauer’s disc diffusion or breakpoints methods according to performance standards of CLSI [24]. For Streptococcus viridians antibiotic sensitivity was carried out using broth dilution for penicillin and disc diffusion method was carried out on Mueller-Hinton Agar (MHA) plates with 5% sheep blood and incubated at 35-37°C with 5-10% CO2 as recommended [24].
Statistical Analysis
The statistical analysis was performed with Stata Statistical Software: Release 13. College Station, TX: StataCorp LP, USA to evaluate the significance of results. For categorical variables, Chi-Square test was used. Significant differences were examined at p-value <0.05 was considered.
Results
The baseline data of the study participants is shown in [Table/Fig-1]. The total number of participants in each group of smokers, EC users and non-smokers was 30. The participants were university students in their 1st to 3rd year of study with a mean age of approximately 20 years (range of 19-21 years). None of the participants had a recent hospital admission, antibiotic use and mouth or URTI which represented exclusion criteria. The table shows that when EC users were asked if they noticed a significant reduction in RTI and early morning phlegm production, all participants answered ‘yes’. The range of nicotine concentration which was labelled by the manufacturer on the E-juice bottle ranged from 3 to 50 mg/mL.
Baseline data of the study participants.
| Study population data | Smokers (n=30) | EC users (n=30) | Non-smokers (n=30) |
|---|
| Sex |
| Male | 30 | 30 | 17 |
| Female | 0 | 0 | 13 |
| Mean age±SD (years) | 20±0.83 | 20.16±1.08 | 20.1±0.82 |
| Range (years) | 19-21 | 19-21 | 19-21 |
| For ECs users: reduction in RTI and morning phlegm production compared to when they were smokers.YesNo | N/AN/A | 300 | N/AN/A |
| The range of nicotine concentration in electronic juice in mg/mL. | N/A | 3-50 | N/A |
RTI: Respiratory tract infections; EC: Electronic cigarette; N/A: Not applicable
Nasal screening results are shown in [Table/Fig-2]. Results showed that a total of 17 Staphylococcus aureus were isolated from all study groups, divided into 16 MSSA (94.1%) and 1 MRSA (5.9%). Difference was only statistically significant in smokers when compared to non-smokers group. The only MRSA isolated in this study was from smokers group but the p-value was not significant when compared to non-smokers group as the control group.
Nasal screening results for Methicillin Susceptible Staphylococcus aureus (MSSA) and Methicillin Resistant Staphylococcus aureus (MRSA) from nonsmokers, smokers and Electronic Cigarette (EC) users.
| Organism sample (n) | MSSA (n, %) +ve/-ve | p-value | MRSA (n, %) +ve/-ve | p-value |
|---|
| Non-smokers (30) | 9 (30)/21 (70) | | 0 (0)/30 (100) | |
| Smokers (30) | 2 (6.7)/28 (93.3) | 0.042* | 1 (3.3)/29 (96.7) | 1.000 |
| EC users (30) | 5 (16.7)/25 (83.3) | 0.360 | 0 (0) / 30 (100) | 1.000 |
*Chi-Square test was used to compare the intergroup difference; *Significant p-value <0.05
[Table/Fig-3] shows the oral screening results among all groups. Results detected 28 Streptococcus viridians and 11 Candida albicans from all groups. Carriage of Streptococcus viridians was highest in non-smokers which was isolated from 14 candidates (46.7%). Difference was only statistically significant in smokers group. Carriage of Candida albicans was detected in 7 (23.3%) smokers, 3 (10%) EC users and in 1(3.3%) non-smokers but there was no statistically significant difference.
Oral cavity screening results for Streptococcus viridians and Candida albicans from Non-smokers, smokers and ECs users.
| Organism sample | Streptococcus viridians s (n, %) +ve/-ve | p-value | Candida albicans (n, %) +ve/-ve | p-value* |
|---|
| Non-smokers (30) | 14 (46.7)/16 (53.3) | | 1 (3.3)/29 (96.7) | |
| Smokers (30) | 5 (16.7)/25 (83.3) | 0.025* | 7 (23.3)/23 (76.7) | 0.052 |
| Electronic cigarette (30) | 9 (30)/21 (70) | 0.288 | 3 (10)/ 27 (90) | 0.612 |
*Chi-Square test was used to compare the intergroup difference; *Significant p-value <0.05
Antibiotic sensitivity results for MSSA, MRSA and Streptococcus viridians are shown in [Table/Fig-4]. It shows that the highest resistance among MSSA isolates was for fusidic acid (43.8%) and erythromycin (38%), followed by trimethoprim-sulfamethoxazole (18.8%), and the least was for mupirocin and linezolid (0%). MRSA was 100% resistant to erythromycin, trimethoprim-sulfamethoxazole and tetracycline but was 100% sensitive to all other antibiotics.
Antibiotic susceptibility pattern of MSSA, MRSA and Streptococcus viridians isolates.
| Nasal bacteria/n | Antibiotic resistance (n %) |
|---|
| E | SXT | CIP | TET | F | MUP | LZD |
|---|
| MSSA/16 | 6 (38) | 3 (18.8) | 2 (12.5) | 1 (6.3) | 7 (43.8) | 0 (0) | 0 (0) |
| MRSA/1 | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Oral bacteria/n | Antibiotic resistance (n %) |
| E | LEV | TET | CC | P | Va | |
| Strep. viridians/28 | 11 (39) | 10 (35.7) | 13 (46) | 3 (11) | 17 (68) | 2 (7.1) | |
MSSA: Methicillin sensitive Staphylococcus aureus; MRSA: Methicillin resistant Staphylococcus aureus; E: Erythromycin; SXT: Trimethoprim-sulfamethoxazole; CIP: Ciprofloxacin; LEV: Levofloxacin; TET: Tetracyclin; F: Fusidic acid, VA: Vancomycin; MUP: Mupirocin; LZD: Linezolid; CC: Clindamycin; P: Penicillin
Streptococcus viridians on the other hand showed the highest resistance to penicillin (68%), and tetracycline (46%). While the least resistance was for vancomycin (7.1%) and clindamycin (11%).
Cultures from EC Juice
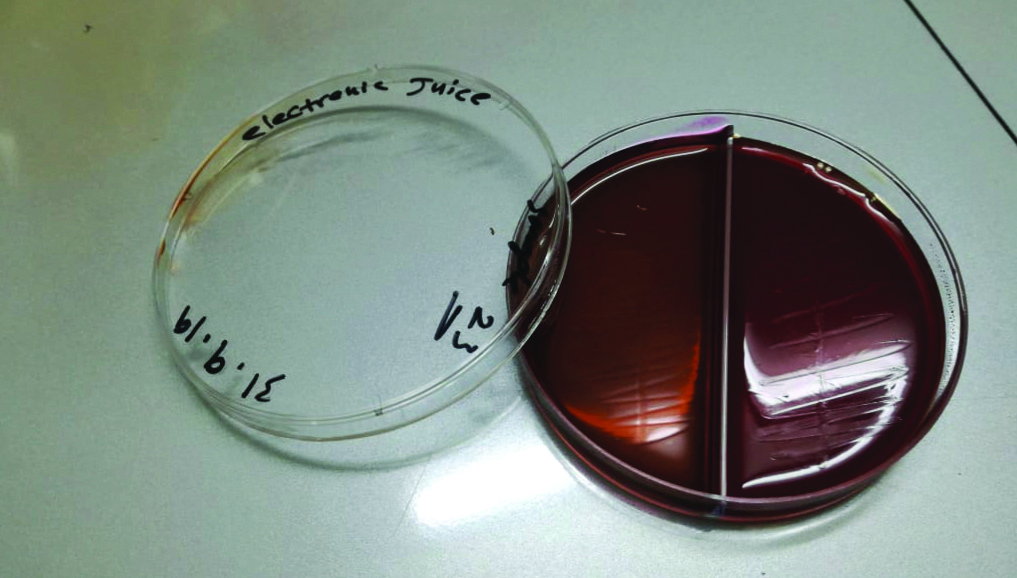
ECs juice samples of each EC user participants were cultured to detect for microbial growth. The samples were obtained from the bottle of the juice directly. All cultures were negative and showed no growth [Table/Fig-5].
Culture of electronic juice on blood and EMB agar showing absence of growth.
Discussion
This pilot study aimed to assess the effects of tobacco smoking and ECs on some of the normal flora and pathogens in nasal and oral cavity in comparison to non-smokers. Isolated organisms were also studied for antibiotic sensitivity to serve as a baseline reference for future therapeutic options and prospective studies. Additionally, the EC juice used by participants was also assessed for possible microbial contamination.
The current study showed that there was no statistically significant difference in the carriage of oral commensal Streptococcus viridians in ECs users compared to the non-smokers as the control group. In contrast, the carriage was lesser among smokers compared to non-smokers. These findings were in agreement with the study by Stewart J et al., who reported that regular use of ECs has no influence on oral bacterial communities as opposed to smoking which significantly affected the bacterial profiles compared to controls [15].
Reduction in normal commensals level would increase the opportunity of pathogenic organisms to replace the normal flora and chances of getting more infections [15,25]. Since the level of the normal interfering oral commensal is not affected in the ECs, it is expected that infections would be lesser among ECs users. This actually was supported by the answers of the ECs users in this study where all of them answered ‘Yes’ to the question about reduction in RTI and phlegm production compared to when they were smokers.
Smoking has been linked to respiratory tract and oral infections [26]. This is mainly because smoking is believed to decrease the oral carriage of Streptococcus viridians as normal flora which has interfering capacity against other pathogenic organism such as Streptococcus pyogenes, Haemophilus influenza and Prevotella spp [15,25]. The present study results are supported by the findings of Brook I and Gober AE on smoking cessation effect on normal flora, who reported that smoking cessation will result in a reversion of nasopharynx flora to normal levels and decreasing risk of infection by pathogenic organisms [25]. This suggests that ECs can be a good means of smoking cessation in terms of good effect on oral flora. Such conclusion is also supported by two recent studies which found that EC had lesser effect on decreasing the commensal oral flora including streptococci spp compared to the dramatic effect of cigarette smoke [14,15].
Smoking and ECs were previously found to significantly increase the mouth carriage rate of Candida spp [27,28]. This was explained by the fact that nicotine is known to facilitate the growth and pathogenesis of Candida spp [28,29]. In this study, the carriage rate of oral Candida albicans not significantly different between smokers and ECs users compared to non-smokers. This is in contrast to what was found by Mokeem A et al., where the oral carriage of Candida albicans was found to be higher among ECs compared to non-smokers [29]. This might be justified by the difference in study population and vaping period. In this study, the average age of population was 20 years and they were using the ECs for 6-8 months, while the age of the other study population was 30 years and they were using ECs for 3.6 years. Additionally, lack of significant difference between groups could be due to a Type II error since this study is a pilot study and it is expected that not all results could reach statistical significance due to small sample size for this pilot phase.
S. aureus is widely distributed in the environment and is carried asymptomatically as normal human flora, specifically located on the skin and nasal area of most healthy individual [30,31]. In the current study, nasal carriage of MSSA showed that non-smokers and ECs users have no statistical difference. In contrast, smokers showed significant decrease in MSSA carriage compared to non-smokers. On the other hand, the carriage of MRSA was not significantly different among all groups. These findings indicate that ECs has no noticeable effect on the MSSA commensals ecology of the nose in tobacco smoking, while the effect of both ECs and tobacco on the carriage rate of the pathogenic MRSA was unremarkable. However, more studies with larger sample size are still mandatory to further explore this relationship.
The overall MRSA rate of isolation among all study population was 3.3% which is slightly lower than what was found in previous studies from Jordan where it ranged from 4 to 7.4% [32-34]. Having a lower rate of MRSA in the current study can be explained by excluding participants with risk factors for MRSA such as recent hospital admission and antibiotic use. Remarkably, all e-juice tested in this study showed no bacterial or yeast growth on selected agars. This is similar to the findings of Varlet V et al., who reported absence of bacterial and yeast growth in 44 bottles of e-juice which were tested for the presence of microorganisms such as gram positive bacteria (S. aureus), gram negative bacteria (Pseudomonas aeruginosa) and yeasts [21].
Antibiotic sensitivity of the isolated organisms showed that the highest resistance in MSSA was for fusidic acid (43.8%) and least for mupirocin and linezolid (0%). On the other hand, MRSA was 100% resistant to erythromycin, trimethoprim-sulfamethoxazole and tetracycline. Antibiotic sensitivity of MSSA and MRSA was comparable to what was previously published in Jordan by Alzoubi H et al., and Al-bakri A et al., where all isolates were found to be sensitive to mupirocin and linezolid. Also, the resistance to fusidic acid of all S.aureus isolates in this study ranged from 0-43.8% compared to 0-100%, and to ciprofloxacin it was 0% for MRSA in this study compared to 0-33.3%, while the MSSA resistance to ciprofloxacin in the current study was slightly higher (12% vs 0%) compared to other studies [33,34].
Streptococcus viridians showed high resistance to penicillin (68%) followed by 46% to tetracyclin and the least was for vancomycin where the rate was 7.1%. Resistance to penicillin is increasing among Streptococcus viridans isolates worldwide, which appears to be as a result of structural changes in the bacterial penicillin-binding protein as was found in a previous study by Doern GV et al., [35] where about 57% of Streptococcus viridians isolates were found not susceptible to penicillin. High resistance rate ranging from 0-16.7 to 43% among Streptococcus viridians isolates to penicillin was also reported by Rozkiewicz D et al., and Salako NO et al., [36,37]. Resistance rate of 46% to tetracyclin found in present study was slightly lower than the 52% rate found also by Rozkiewicz D et al., [36]. Low level of vancomycin resistance (7.1%) was detected in the current study which was lower than the 12% and 4% rate published previously by Abu-zineh R et al., and Salako NO et al., respectively [18,37]. Differences in sample size and in Streptococcus viridans isolates among the studies can be the reason for such variance in resistance rate, however, the overall susceptibility to vancomycin is still considered high.
The market of ECs is new and growing. Therefore, studies are still needed to determine its effects on health. The current study highlighted the effect of ECs on some oral and nasal commensals which showed the absence of significant alteration of the microbial profile by ECs compared to tobacco smoking. In order to assess the safety of ECs as an alternative to tobacco smoking, other different end points effects should be studied to determine its impact on human health and to help in adopting the appropriate policies for ECs use.
Limitation(s)
There were some potential limitations of the current study. It was carried out in only one area of Jordan because of a lack of resources, which might limit the results to a certain region. This study was a pilot study and some positive results have been shown, and it is expected that not all results could reach statistical significance due to small sample size for this pilot phase. Additionally, females who were either smokers or using ECs refused to participate in the study for social and privacy reasons, which limited the role of gender as a factor during statistical analysis.
Conclusion(s)
The ECs did not statistically reduce the carriage of MSSA, S.viridans commensals in comparison to smoking which significantly decreased the carriage rate of both commensals which could predispose the host to be colonisation with more virulent pathogens. This suggests that ECs might be a safer alternative for tobacco smoking cessation in terms of its effect on microbiota. Indeed, further studies in large multi-location cohorts is mandatory to ascertain the effects of ECs on oro-nasal flora and across the respiratory sites and to assess the effects on other human health aspects.
RTI: Respiratory tract infections; EC: Electronic cigarette; N/A: Not applicable
*Chi-Square test was used to compare the intergroup difference; *Significant p-value <0.05
*Chi-Square test was used to compare the intergroup difference; *Significant p-value <0.05
MSSA: Methicillin sensitive Staphylococcus aureus; MRSA: Methicillin resistant Staphylococcus aureus; E: Erythromycin; SXT: Trimethoprim-sulfamethoxazole; CIP: Ciprofloxacin; LEV: Levofloxacin; TET: Tetracyclin; F: Fusidic acid, VA: Vancomycin; MUP: Mupirocin; LZD: Linezolid; CC: Clindamycin; P: Penicillin