Electrochemical Sensing for Examining Vitamin D3 based on MIP using NOVA 1.7 and Autolab PGSTAT 302N
Saveri Singh1, Naresh Batra2, MA Ansari3, Shabana Urooj4
1 Scholar, Department of Electrical Engineering, School of Engineering, Gautam Buddha University, Greater Noida, Uttar Pradesh, India.
2 Scholar, Department of Electrical Engineering, School of Engineering, Gautam Buddha University, Greater Noida, Uttar Pradesh, India.
3 Assistant Professor, Department of Electrical Engineering, School of Engineering, Gautam Buddha University, Greater Noida, Uttar Pradesh, India.
4 Associate Professor, Department of Electrical Engineering, College of Engineering, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shabana Urooj, Department of Electrical Engineering, School of Engineering, Gautam Buddha University, Greater Noida-201312, Uttar Pradesh, India.
E-mail: shabanaurooj@ieee.org; smurooj@pnu.edu.sa
Introduction
An electrochemical sensor has the ability to transform the associated data containing electrochemical reactions into a reliable representative signal. The electrochemical sensors can be classified into potentiometric, conductometric, and ampere-metric or Volta-metric. Although, there are various electrochemical techniques for the detection of Vitamin D3, there is still a need for a simplified and cost-effective method. An electrochemical sensor provides great sensitivity towards the detection of the analyte.
Aim
To fabricate an electrochemical sensor for the detection of Vitamin D3. The sensor used Molecular Imprinted Polymer (MIP) based Screen Printed Carbon Electrode (SPCE).
Materials and Methods
The SPCE used was a three-electrode system consisting of silver working electrode, silver reference electrode and a counter carbon electrode. The reagents used in the experiment was p-Phenylenediamine, resorcinol and Vitamin D3 that were applied in a particular amount onto the SPCE. The process of electropolymerisation was carried out in order to form a non-conductive layer. Cavities were gradually formed on the surface of SPCE. A mediator was used to obtain reliable results for the detection of Vitamin D3. It is evident from the existing literature that the number of scans of electropolymerisation holds a significant role in this process. The procedure was applied for the formation of non-imprinted electrode in the absence of the analyte.
Results
The presence of the template i.e., Vitamin D3 was recorded using the developed electrochemical sensor. The current decreased on rebinding of Vitamin D3 which resulted in the change of redox peak of ferricyanide. This signified the sudden increase in concentration of Vitamin D3 specifying its presence.
Conclusion
The results obtained specifies the great sensitivity of the electrochemical sensor towards the template i.e., vitamin D3. The clinical relevance of such electrochemical sensors is that they produce simple, accurate and reproducible results which can be used to optimise the care of patients.
Electropolymerisation, Molecular imprinted polymer, Screen printed carbon electrode, Voltammetry
Introduction
A device that converts an electrochemical data into a signal that can be analysed and used is known as an electrochemical sensor. These sensors mainly consist of two components i.e. a chemical recognition system and a physiochemical transducer. The chemical recognition system plays the most significant role in the sensor functionalisation while the physical transducer transforms chemical signal into a detectable signal. A sensing electrode is formed as the two components are combined together. On the other hand, biosensors are the devices that use biochemical mechanism for transforming the signal data [1].
Lower level of vitamin D are found in new born infants that are reliant on breast milk, supplements or sunlight as causes of vitamin D within the initial months [2]. The vitamin D content of breast milk depends on the status of the maternal vitamin D which is usually low. Exposure to the sun could be a constraint for the people living at high latitudes. Lack of Vitamin D in infants may cause rickets and difficulty in breathing.
The 25-hydroxyvitamin D {25(OH)D} is found in abundance in human body with almost 95-99% bound to the Vitamin D Binding Proteins (VDBP) [3]. This restricts the immunoassay linking during the detection tests [4]. Measurement of vitamin D3 is quite significant in climates at higher latitudes with limited sunlight. Majority of people at such places are found to be deficient of Vitamin D [5]. Amongst all the vitamin groups, vitamin D is important for the development of muscles and bones. According to a 2019 pan-India study, 70-90% Indians is deficient of vitamin D which could cause repercussions like underdeveloped bone structure in babies and bone disease in adults.
A report stated that 42% of the US population is generally left undiagnosed which in a long term can cause severe bone diseases [6]. The tests for determining Vitamin D3 are majorly based on laboratories experiments which have high costs. Therefore, there is a requirement of a cost-effective method for determining Vitamin D3. A 25hydroxyvitamin D3{25(OH)D3}, is the most widely used biomarker but due to the complex structure it becomes difficult to use the biomarker [7].
As 25-hydroxyvitamin D {25(OH) D} is the foremost metabolite of Vitamin D3 which is found in abundance, therefore, recognition of 25(OH)D concentration is the utmost approachable way to determine the vitamin D quantity in plasma or serum. A precise and reliable result can be obtained by utilising the voltametric procedures. It can be assured by covering a significant range. Imprinted polymers are being used as improving agents in fabricating an enormously selective voltametric sensor [8].
The most common way for improving the selectivity of electrode is the modification of the electrode with the use of polymers that have ability to adsorb the template from the surface of electrode. Molecular imprinting is a greatly equipped and advanced version for synthesising polymers. The major limitation of conventional methods that are used for detection is the poor limit of detection.
Currently, the evaluation of liquid chromatography is quite an alternative to the traditional techniques [9], however the method uses a great amount of analyte and calls for specialised high-priced equipment [10]. Furthermore, there appears to be variability between related techniques [11-15] used for D3 detection. Therefore, there is a need for the development of method that can easily and readily detect vitamin D. In this work the authors seek out to detect presence of Vitamin D on a sample of SPCE that has been electropolymerised, bounded by Vitamin D and finally rebounded to detect the presence of Vitamin D.
Molecularly Imprinted Polymer (MIP) based Polymerisation [
Table/Fig-1]
Molecular imprinting of polymer.
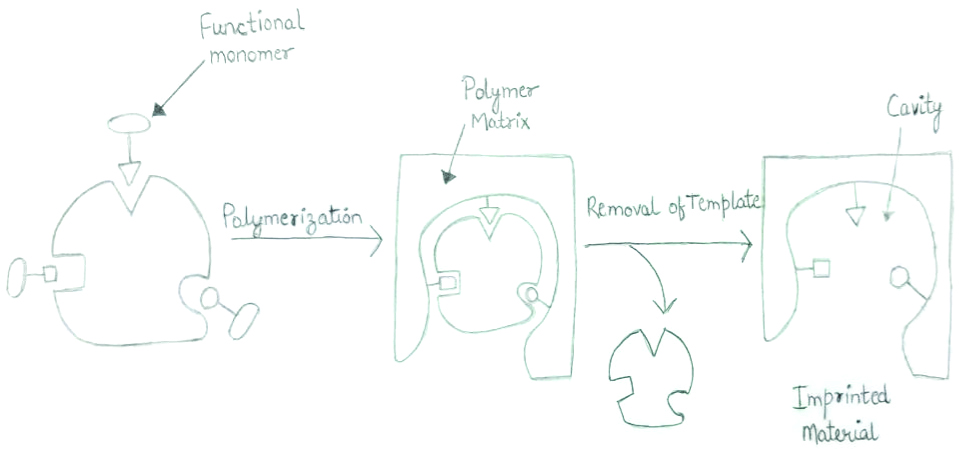
Polymerisation based on molecular imprinting is a method used since a long time back. It is an alternative to the conventional methods that are being used. The MIP are commonly known as “plastic antibodies” having the capability to lock the particular analyte from a matrix. They act as the receptors for target molecule. A functional monomer, cross-linker, initiator along with target molecule are involved while a standard polymerisation based on MIP reaction is taking place. Initially, a pre-polymerisation is produced as the analyte is linked to the monomer via bonds (covalent bond, semi-covalent, or metal coordinated bond). The bonds having non-covalent properties are flexible enough to allow it to be used in the preparations of MIP. As the analyte is being removed from the complex matrix, cavities are created having particular shape and structure. These act as linking sites after they are attached again for detection [8].
MIP are synthetic polymeric materials that show sites selectively that are formed at the time of polymerisation. The monomers involve with the molecule resulting in the formation of a complex [16]. This particular step is significant as exceptional interactions among these that can affect the selectivity as well as the pattern of recognition within the ultimate form of polymer [17].
Materials and Methods
The experiment was carried out at Gautam Buddha University, Biomedical and Virtual Instrumentation laboratory in December 2019, with the permission of competent authority.
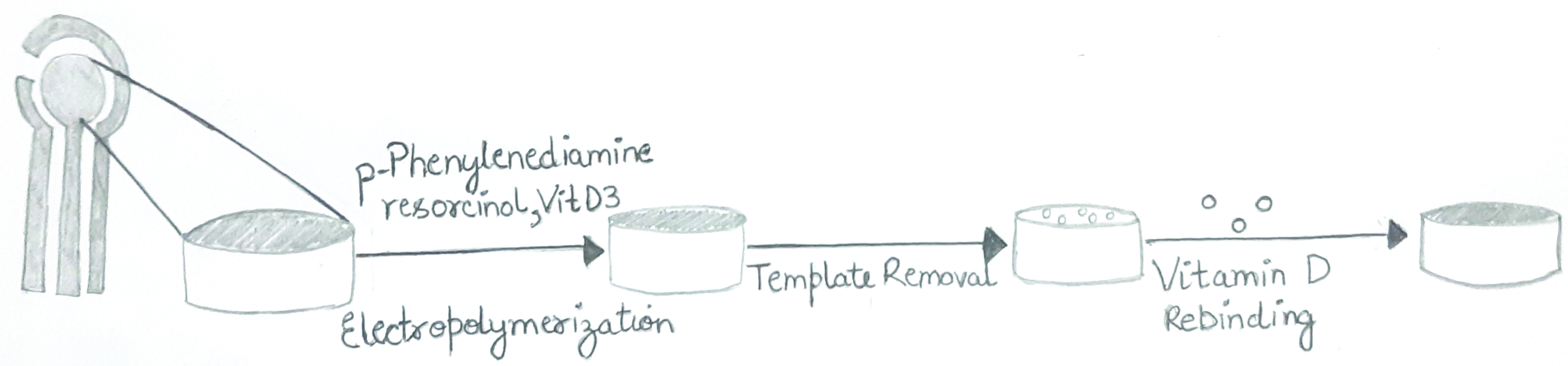
The SPCE was supplied form Metrohm DropSens (India) having the parameters of 3.5 cm (length) × 1.0 cm (width) × 0.05 cm (height). The SPCE consisted of three electrode system i.e., a silver working electrode, silver reference electrode and a counter carbon electrode. The potentiostat was purchased form Metrohm (India) of the model PGSTAT302. The chemicals p-Phenylenediamine, resorcinol and vitamin D3 were supplied from Sigma-Aldrich (India). The mixture of p-Phenylenediamine and resorcinol were placed on the SPCE including the analyte Vitamin D3. Electropolymerisation of the functional monomers created a non-conductive surface that eventually reduced the shifting of electrons on the electrode surface. Cavities that were formed at the surface were removed on the removal of the analyte. This resulted in the electrons shifting once again. A mediator (ferricyanide) purchased from Sigma-Aldrich, was added as the oxidation peak of Vitamin D3 is quite weak and was unable to show reliable results [Table/Fig-2].
Schematic representation of the sensor production process.
Experiment
Electrochemical data was recorded using a potentiostat accompanied with a three-electrode system. The carbon electrode had the diameter of 4 mm and acted as the working electrode, silver (Ag) was the reference electrode and a counter electrode was present which was a carbon electrode.
a. Electrochemical Process
A 4mM of p-Phenylenediamine, 4mM of resorcinol and 0.35 mM of Vitamin D3 was mixed in 70% methanol solution that consisted of 100 mM acetate buffer. The pH of the mixture was kept at 5.2. While the monomers undergo the process of electropolymerisation, a non-conductive film was created at the surface of electrode. CV was applied within a range of 0-0.8V as 30 μL of the solution was delivered on the electrode surface. The process of electropolymerisation was repeated for seven cycles at a rate of 50 mV/s. The procedure was applied for the formation of non-imprinted electrode in the absence of the analyte [Table/Fig-3] [8].
b. Autolab PGSTAT 302N
Autolab PGSTAT 302N is an excessive modem that act as a potentiostat/galvanostat. It comprises of a bandwidth of 1 MHz and a compliance voltage of 30 V mixed with FRA32M module. It is particularly designed for electrochemical impedance spectroscopy. It is an advanced version of the PGSTAT30. The maximum amount of current that it can work with is 2A, which can be increased using BOOSTER20A [Table/Fig-4] [18].

c. NOVA 1.7
NOVA is an electrochemistry-based software originated from Metrohm Autolab. The software is used to program Autolab instruments. NOVA offers flexibility to the Autolab equipment. The designing of the software is simple yet powerful. The subtle interface provides a user- friendly platform to work on [19].
d. Optimisation
Electropolymerisation of the functional monomers creates a non-conductive surface that eventually reduces the shifting of electrons on the electrode surface. Cavities that are formed at the surface are removed on the removal of the analyte. This results in the electrons shifting once again. A mediator (ferricyanide) is added as the oxidation peak of Vitamin D3 is quite weak and is unable to show reliable results. As the analyte is rebounded onto the cavity, the redox peak of ferricyanide is decreased. The decreased redox peak of mediator is varied linearly with the concentration of Vitamin D3. The major parameter in the process is the number of cycles of electropolymerisation [4]. A 10 μL of the template i.e., Vitamin D is kept on the surface of analyte imprinted SPCE and the incubation time being 15 minutes at Standard Temperature and Pressure (STP) (25°C). The surface is cleaned using 20 μL of washing agents (water and acetonitrile solution) for three times. This results in removing the unbounded analyte. The 10 μL of 10 mM of mediator solution (ferricyanide) on modified surface of SPCE was applied. Cyclic Voltammetry (CV) was activated at a scan rate of 50 mV/s. It works in a range between 0.2 to 0.8V.
Results
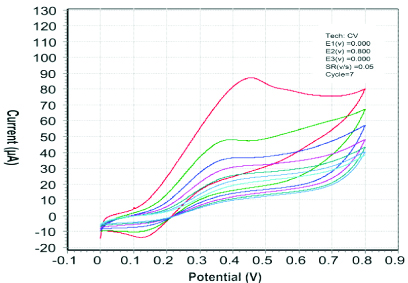
In [Table/Fig-5], the graph shows results of seven cycles during electropolymerisation. The number of cycles used in the process was the optimised number of scans, according to the available literature. If the cycles of electropolymerisation were increased more than the optimised value, the removal of template became difficult. Therefore, the experiment was carried out with seven cycles. With all the cycles (scans) shown in the figure, the current decreased and approached zero. This signified the development of a non-conductive film on the surface of electrode. The potential involved was between 0-0.8 V.
Ideal experiment showing CV curves during electropolymerisation on the surface of SPCE; the topmost red curve of CV signifies the first scan of electropolymerisation process and the successive curves; green, blue, pink and so on curves plunging with current up to next six scans of electropolymerisation.
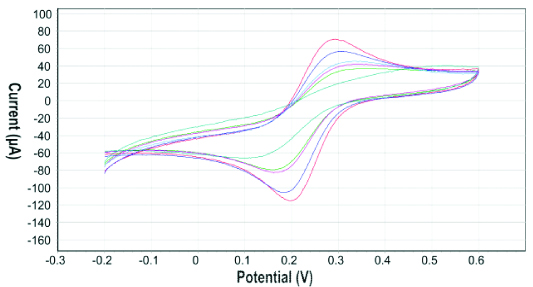
In [Table/Fig-6], the redox peak of the mediator i.e., ferricyanide gets reduced as the template is rebounded on the surface of the SPCE. It can be clearly observed from the recorded signals that the redox peak of the ferricyanide varies as the concentration of the vitamin D3 changes. The signal is suppressed as the template is rebounded on the surface of SPCE. The current reduces as the concentration of vitamin D3 is increased from 1 nM to 100 nM.
CVs on rebinding of vitamin D resulting in the decrease redox peak of ferricyanide; the outermost red curve of CV signifies the first scan of rebinding of Vitamin D3 on the SPCE. The successive curves blue, turquoise, pink and so on with decreasing current represents the subsequent scans of rebinding of template-Vitamin D3.
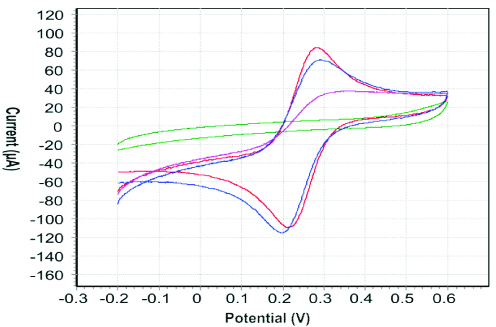
In [Table/Fig-7] the red curve signifies the CV representing a SPCE with no functionalisation (bare). The current reaches maximum peak in this case as there is no resistance due to a non-conductive film. The graph shows CV representing ideal curve after electropolymerisation on the surface of SPCE. The blue curve represents the CV on the removal of the template i.e., vitamin D3. The curve in pink signifies the CV when the template is rebounded on the surface of SPCE. The same process was repeated for non-imprinted SPCE surface but no signal was developed. Results are recorded by using the NOVA 1.7 software which was interfaced with impedance analyser AUTOLAB PGSTAT 302N. The results show the high sensitivity of the SPCE towards the template.
The uppermost red curve represents the CV of bare SPCE, green curve of CV signifies the surface of SPCE after electropolymerisation, the blue curve represents the surface of SPCE after Vitamin D3 removal, the pink curve demonstrates the CV of SPCE after Vitamin D3 rebinding.
Discussion
In this study Vitamin D3 on the surface of SPCE using MIP has been detected. Presently, the evaluation of liquid chromatography is the alternative to the superseded practices [9], though the method uses a great amount of analyte and demands specialised high-priced apparatus [10]. A report stated that the detection of vitamin D using paper based microfluidic system. It involved a colorimetric method involving gold nanoparticle-based immunoassay having a concentration peak shift from 520 nm to 650 nm due to clustering of gold nanoparticles [3]. Another study reported a method for detection of Vitamin D3 using gadolium oxide nano-rods having the limit of detection of 0.10 ng/mL [20]. The Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS), reported that a normal spectrum of Vitamin D should be 30-50 ng/mL on the basis of the diagnosis and management of severe bone diseases like osteoporosis [21]. However, a recent study suggested that the normal range should vary between 20-30 nm/mL. Any value above and below the range could affect liver, bones, obesity, kidney and pregnancy. The governance and management of the medical and clinical applications need standard methodologies that could be accurate, cost effective and reliable. This could result in optimisation and betterment of care for the patients in medical activities [22]. A study reported the normal value of vitamin D3 to be above 20 ng/mL [23]. It is evident form the literature that approximately 50% of young people in Italy with sound health are deficient of Vitamin D, moreover, insufficiency increases especially during the winters and old age [24]. Thus, this would help in improving accurate, reproducibility in patient care. High-Pressure Liquid Chromatography with UV detector (HPLC-UV) or Chemiluminescent Immunoassay (CLIA) based techniques are considered as simple techniques which are easy to use, rapid and of great quality [25].
Limitation(s)
There are various advantages of using an electrochemical sensor for the detection of vitamin D3 but there are some limitations too. It is difficult to optimise the number of scans of electropolymerisation process as removal of thick non-conductive film on the SPCE is impossible. The negative potential can be used with a number of limitations while using CV. In this study, the limit of the negative potential is -0.2V.
Conclusion(s)
The CV is applied during electropolymerisation of the surface of SPCE. The current readily decreases as the electropolymerisation is processed and ultimately approaches zero due to the formation of non-conductive film on the surface of the SPCE. The obtained signal is recorded as decreasing on rebounding. Furthermore, human plasma can be collected and concentration of Vitamin D3 can be measured. The determination of calibration work can be performed for different concentration levels with vitamin D3, detection of zinc in plasma can be done by using MIP based electrochemical sensor. Detection of vitamin B12 in real samples of human plasma can also be performed by using MIP based electrochemical sensor. Electrochemical sensors based on MIP can be further used for assessing bioactive compounds.
Author Declaration:
Financial or Other Competing Interests: As declared above
Was Ethics Committee Approval obtained for this study? NA
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 17, 2020
Manual Googling: May 30, 2020
iThenticate Software: Jun 24, 2020 (14%)
[1]. Farnoush F, Gupta V, Zamani H, Electrochemical sensors and biosensorsInternational Journal of Electrochemistry 2011 10.4061/2011/352546 [Google Scholar] [CrossRef]
[2]. Hollis BW, Comparison of commercially available (125)-I based RIA methods for the determination of circulating 25-hydroxyvitamin DClin Chem 2000 46(10):1657-61.10.1093/clinchem/46.10.165711017946 [Google Scholar] [CrossRef] [PubMed]
[3]. Weng CH, Chou HY, Chen MH, Lin YS, A portable platform for the quantification of Vitamin D levels by using paper-based microfluidic11th Biomedical Engineering International Conference BMEiCON-2018 2018 :1-3.10.1109/BMEiCON.2018.8609988PMC6438165 [Google Scholar] [CrossRef] [PubMed]
[4]. Vitamin D testing limit a mistake: MD. 2010. doi: http://www.cbc.ca/news/health/story/2010/10/04/vitamin-d-testing.html [Google Scholar]
[5]. Vitamin D blood levels of Canadians. 2013. doi: http://www.statcan.gc.ca/pub/82–624-x/2013001/article/11727- eng.htm [Google Scholar]
[6]. Parvane R, Tadepalli S, Singh P, Qian A, Joshi R, Kandala H, Prevalence of Vitamin D deficiency and associated risk factors in the US population (2011-2012)Cureusdoi:10. 10.7759/cureus.2741 [Google Scholar]
[7]. Vemulapati S, Rey E, O’Dell D, Mehta S, Erickso D, A quantitative point-of-need assay for the assessment of vitamin d3 deficiencyScientific Reports 2017 7(1):1414210.1038/s41598-017-13044-529074843 [Google Scholar] [CrossRef] [PubMed]
[8]. Solmaz K, Shahriyar B, Shahab B, A novel electrochemical sensor based on plastic antibodies for vitamin D3 detection in real samplesIEEE Sensors Journal 2019 19(13):01-01.10.1109/JSEN.2019.2903090 [Google Scholar] [CrossRef]
[9]. Bruce SJ, Bertrand R, Alexandre B, Benoït P, Idris G, Olivier B, Analysis and quantification of vitamin D metabolites in serum by ultra-performance liquid chromatography coupled to tandem mass spectrometry and high-resolution mass spectrometry-A method comparison and validationRapid Communication, Mass Spectrometr 2012 27(1):200-06.10.1002/rcm.643923239334 [Google Scholar] [CrossRef] [PubMed]
[10]. He CS, Gleeson M, Fraser WD, Measurement of circulating 25-hydroxy vitamin D using three commercial enzyme-linked immunosorbent assay kits with comparison to liquid chromatography: Tandem mass spectrometry methodISRN Nutrition 2013 2013:72313910.5402/2013/72313924967259 [Google Scholar] [CrossRef] [PubMed]
[11]. Binkley N, Lensmeyer G, 5-hydroxyvitamin D assays and their clinical utility in Vitamin D: Physiology, Molecular Biology, and Clinical Applications (Nutrition and Health), M.F. Holick, Ed. 2010 2nd edNew York, NY, USASpringer:383-99.10.1007/978-1-60327-303-9_19 [Google Scholar] [CrossRef]
[12]. Marcelo PC, Sophie GB, Martial H, Colette B, Franck V, Philippe S, Comparison between UV index measurements performed by research-grade and consumer-products instrumentsPhotochemical & Photobiological Sciences: Official Journal of the European Photochemistry Association and the European Society for Photobiology 2010 :459-63.10.1039/b9pp00179d20354638 [Google Scholar] [CrossRef] [PubMed]
[13]. Moreno P, Salvadó V, Determination of eight water and fat-soluble vitamins in multivitamin pharmaceutical formulations by high performance liquid chromatographyJournal of Chromatography 2000 870:207-16.doi:10.1016/S0021-9673(99)01021-310.1016/S0021-9673(99)01021-3 [Google Scholar] [CrossRef]
[14]. William B, Jake E, Susan G, James H, Ronald H, Kristine P, Liquid chromatography with ultraviolet and dual parallel mass spectrometric detection for analysis of vitamin D in retail fortified orange juiceJournal of Food Composition and Analysis 2011 (24):299-306.10.1016/j.jfca.2010.09.020 [Google Scholar] [CrossRef]
[15]. Chatzimichalakis PF, Samanidou VF, Papadoyannis IN, Development of a validated liquid chromatography method for the simultaneous determination of eight fat-soluble vitamins in biological fluids after solid-phase extractionJournal of chromatography B-Anal. Technol. Biomedical Life Sciences. vol 2004 805(2):289-96.10.1016/j.jchromb.2004.03.00915135103 [Google Scholar] [CrossRef] [PubMed]
[16]. Haupt K, Linares AV, Bompart M, Bui BTS, Molecularly Imprinted PolymersIn Molecular Imprinting-Topics in current chemistry 2012 325Berlin, GermanySpringer:1-28.10.1007/128_2011_30722183146 [Google Scholar] [CrossRef] [PubMed]
[17]. Bernadette TSB, Karsten H, Molecularly imprinted polymers: Synthetic receptors in bioanalysisAnalytical and Bioanalytical Chemistry 2010 398:2481-92.10.1007/s00216-010-4158-x20845034 [Google Scholar] [CrossRef] [PubMed]
[18]. AutolabPGSTAT302N-High Performance. 2010; doi: https://www.metrohm-autolab.com/Products/Echem/NSeriesFolder/PGSTAT302N [Google Scholar]
[19]. NOVA: Advanced Electrochemical Software. 2010; doi: https://www.ecochemie.nl/Products/Echem/Software/Nova.html [Google Scholar]
[20]. Chauhan D, Kumar R, Panda A, Solanki P, An efficient electrochemical biosensor for Vitamin-D3 detection based on aspartic acid functionalised gadolinium oxide nanorodsJournal of Materials Research and Technology 2019 8(6):5490-503.10.1016/j.jmrt.2019.09.017 [Google Scholar] [CrossRef]
[21]. Cesareo R, Attanasio R, Caputo M, Castello R, Chiodini I, Falchetti A, Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) position statement: Clinical management of Vitamin D deficiency in adultsNutrients 2018 10:54610.3390/nu1005054629702603 [Google Scholar] [CrossRef] [PubMed]
[22]. Poscia A, Cambieri A, Tucceri C, Ricciardi W, Volpe M, Audit as a tool to assess and promote the quality of medical records and hospital appropriateness: Methodology and preliminary results, IgSanita Pubbl 2015 71:139-56. [Google Scholar]
[23]. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to knowJ Am Diet Assoc 2011 111(4):524-27.10.1016/j.jada.2011.01.00421443983 [Google Scholar] [CrossRef] [PubMed]
[24]. Adami S, Romagnoli E, Carnevale V, Linee guida su prevenzione e trattamento dell’ipovitaminosi D con colecalciferolo. SIOMMMS [Guidelines on prevention and treatment of vitamin D deficiency. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS)]Reumatismo 2011 63(3):129-47.10.4081/reumatismo.2011.12922257914 [Google Scholar] [CrossRef] [PubMed]
[25]. Albolino S, Bellandi T, Cappelletti S, Di Paolo M, Fineschi V, Frati P, New rules on patient’s safety and professional liability for the Italian Health ServiceCurr Pharm Biotechnol 2019 20(8):615-24.10.2174/138920102066619040809401630961486 [Google Scholar] [CrossRef] [PubMed]