Parkinson plus syndrome is a group of degenerative neurological disorders which includes PSP, MSA and Corticobasal Ganglionic Degeneration (CBGD) [1]. Diagnosis of each condition is important since it affects patient’s management, rehabilitation and prognosis. Even though it differs from classical PD in clinical features and response to levodopa; during the early course of illness clinical diagnosis can be challenging [2].
Various imaging features are described in PSP. The characteristic MRI findings in PSP include atrophy of midbrain with dilatation of the third ventricle, reduced midbrain anteroposterior diameter (<17 mm), flattening/concavity of the superior midbrain and atrophy of tegmentum on axial sections (Morning glory sign) [3,4]. Reduction of the anteroposterior midbrain at the level of superior colliculi gives the mickey-mouse sign on the axial images. Midbrain atrophy along with preservation of pons gives rise to the hummingbird sign, which is also referred to as the penguin sign [5,6]. The tegmentum represents the head with a thin beak and pons represents the body of humming bird/penguin. Other imaging features include: atrophy and Fluid Attenuated Inversion Recovery (FLAIR) hyper intensity of the superior cerebellar peduncles. These characteristic features help to differentiate PSP from MSA and Parkinsonism [7,8]. MRI is especially a useful tool in parkinsonian syndromes, since it identifies changes produced by the neurodegeneration. Rostral midbrain atrophy is seen in PSP and pontine atrophy is seen in MSA. Midbrain pons ratio helps to identify the atrophy involving midbrain, thus helps in differentiating PSP from other causes of parkinsonism [2]. In that scenario, usefulness of MRI by assessing midbrain pons ratio in differentiating PSP from other Parkinson plus syndrome were studied. A part of study was published on usefulness of pontine diameter in MSA [9]. The same cohort was used in the present study for assessing Midbrain diameter and Midbrain pons ratio.
Materials and Methods
This cross-sectional study was conducted in Department of Neurology, Govt. Medical College, Kottayam, Kerala, India from January 2017 to June 2018. This study was approved by scientific research committee and Institutional Review Board (IRB NO-151/2017). Sample size was calculated by using formula where, Zα/2 1.96, (Z1-β) 0.842, σ2 is the standard deviation and d margin of error [10]:
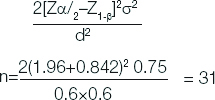
Total size required for all groups=4×31=124. 124 patients includes 30 patients in each group (PSP, MSA, PD, controls) and four patients of CBGD.
Inclusion criteria: Patients with clinical features of PSP, MSA, CBD, Parkinson’s disease and control population who has taken MRI for other reasons (Headache, epilepsy) attending Neurology department were enrolled in the study.
Exclusion criteria: Vascular Parkinsonism and Drug induced parkinsonism and patients on psychiatric medications were excluded.
Criteria
PSP [11]- Criteria for diagnosis
Disease onset 40 years or late.
Gradually progressive disorder.
Vertical supranuclear palsy/slowing of vertical saccades.
Prominent postural instability with tendency to fall in the first year of disease onset.
No evidence of other diseases that could explain the foregoing features, as indicated by mandatory exclusion criteria.
MSA [12]- Clinical criteria
Onset >30 years with progressive course.
Autonomic failure -urinary incontinence.
Orthostatic Hypotension- decrease of blood pressure at least 30 mmHg systolic or 15 mmHg diastolic blood pressure on 3 minutes of standing.
Poorly levodopa-responsive parkinsonism (bradykinesia with rigidity, tremor, or postural instability).
Gait ataxia with cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction.
Corticobasal Degeneration (CBD) [13]
Asymmetric presentation of two of the below features:
1) Limb rigidity or akinesia; 2) asymmetrical limb dystonia; 3) limb myoclonus.
Plus two of the below features-
a) Orobuccal or limb apraxia; b) alien limb phenomenon; c) cortical sensory deficit.
MRI Protocol
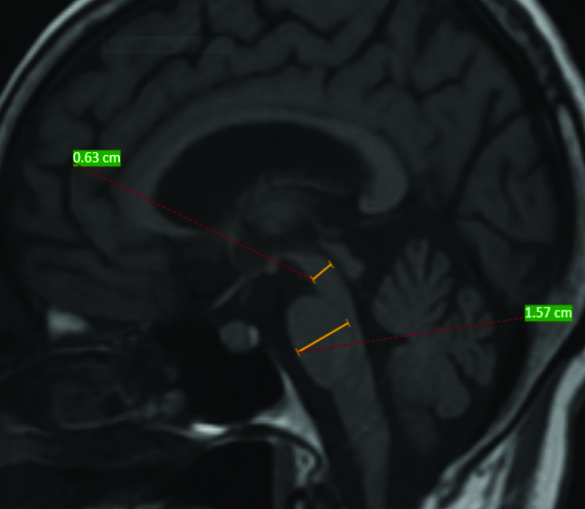
MRI was performed using 1.5 Tesla machine using a standard receive and transmit head coil. MRI was reported by the radiologist who was blinded to clinical diagnosis. Midbrain diameter and pontine diameter was calculated using mid-sagittal T1 image. Pontine diameter was taken without pontine tegmentum. Similarly, midbrain measurement was taken without collicular plate. For that a line was drawn from superior to inferior part of pons and midbrain considering as an ellipse. Another line was drawn with maximal measurement perpendicular to initial line and was taken as diameter of midbrain and pons. Measurement was done according to Massey LA et al., [14]. Measurement in the patient is demonstrated in [Table/Fig-1].
Demonstration of measurement of midbrain diameter (shorter line) (0.63 cm) and Pontine diameter (1.57 cm) in the patient-(Midbrain pons ratio was 0.40).
Statistical Analysis
Microsoft excel sheet and Statistical Package for the Social Sciences (SPSS) software V 20 were used for entering and analysing the data, respectively. Quantitative analysis was done using ANOVA with post-hoc Tukey correction for various groups.
Results
Out of 30 patients in each group (PSP, MSA, PD, Controls) 20 were males and 10 were females and four patients of CBD were males. Mean age of patients in PSP was 59.47 with a standard deviation of 3.86. Mean age of onset in MSA was 58.63 years with a SD of 3.891 [Table/Fig-2]. Mean disease duration in PSP was 2.97 years with a SD of 1.035 [Table/Fig-3].
| Diagnosis | N | Mean (years) | SD |
|---|
| PSP | 30 | 59.47 | 3.86 |
| MSA | 30 | 58.63 | 3.89 |
| PD | 30 | 63.17 | 3.27 |
| Control | 30 | 61.10 | 3.97 |
| CBD | 4 | 60.00 | 2.16 |
| Total | 124 | 60.47 | 3.43 |
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
| Diagnosis | N | Mean (years) | SD |
|---|
| PSP | 30 | 2.97 | 1.035 |
| MSA | 30 | 3.56 | 1.193 |
| PD | 30 | 3.90 | 1.233 |
| Control | 30 | 3.62 | 1.134 |
| CBD | 4 | 2.75 | 0.957 |
| Total | 124 | 1.184 | 0.402 |
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
Mean midbrain diameter in PSP was 7.8 mm with a SD of 0.83. Mean midbrain diameter in MSA was 10.96 with a SD of 0.74 [Table/Fig-4].
| Diagnosis | Mean (mm) | SD |
|---|
| PSP | 7.8 | 0.83 |
| MSA | 10.96 | 0.74 |
| PD | 11.16 | 0.52 |
| Control | 11.57 | 0.52 |
| CBD | 10.98 | 0.17 |
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
Mean midbrain to pons ratio in PSP was 0.4555 with a SD of 0.033 [Table/Fig-5].
| PSP | MSA | PD | Control | CBD | p-value |
|---|
| 0.45±0.033 | 0.69±0.06 | 0.63±0.035 | 0.64±0.02 | 0.62±0.02 | PSP < MSA, PD, CBD, Control (p<0.001) |
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration
ANOVA was done for quantitative variable with more than three groups and post-hoc Tukey analysis was done to evaluate the p-value. Statistical analysis using ANOVA showed statistically significant difference between the group with respect to midbrain diameter and midbrain pons ratio [Table/Fig-6]. Post-hoc Tukey analysis showed statistically significant difference in midbrain diameter between PSP and other groups was obtained after post-hoc Tukey analysis (p<.001).
Comparison of midbrain diameter and midbrain pons ratio between various groups using ANOVA and Post-hoc Tukey.
| | Sum of squares | df | Mean square | F | Sig. |
|---|
| Midbrain diameter | Between groups | 261.301 | 4 | 65.325 | 148.576 | 0.001 |
| Within groups | 52.321 | 119 | 0.440 |
| Total | 313.622 | 123 | | | |
| Pontine diameter | Between groups | 149.389 | 4 | 37.347 | 29.704 | 0.001 |
| Within groups | 149.621 | 119 | 1.257 |
| Total | 299.011 | 123 | | | |
| Midbrain ponsratio | Between groups | 0.992 | 4 | 0.248 | 135.648 | 0.001 |
| Within groups | 0.218 | 119 | 0.002 |
| Total | 1.209 | 123 | | | |
Discussion
In PSP patients imaging appearance as a result of atrophied segment of midbrain has decreased sensitivity with interobserver variability. So quantitiative assessment was done to increase the sensitivity in the present study. Most of the PSP patients have onset in 7th decade of life. In present study, mean age of onset of PSP was 59 years; which was comparable to that found in other study [4]. Average duration of the disease in PSP was 2.97 years at the time of scan.
Mean midbrain diameter in PSP was found to be 7.8+0.83 mm. Mean midbrain diameter was found to be lower in PSP when compared to other groups and was statistically significant (p<0.001). A landmark study done by Massey LA et al., looked in to the midbrain pons ratio in patients with PSP [14]. The strength of the study done by Massey LA et al., lies in the fact that they also analysed midbrain pons ratio in definite cases of PSP which by definition included pathologically proven cases. Twelve pathologically proven cases and 21 clinically diagnosed cases of PSP were analysed in the study by Massey LA et al., mean midbrain diameter in the pathologically diagnosed group was 8.1 (1.2). Mean midbrain diameter in clinically diagnosed group was 7.55 (1.12). Massey LA et al., proposed that midbrain diameter of less than 9.35 mm is 100% specific for the diagnosis of PSP. Midbrain diameter in pathologically proven cases of PSP was less than 9.35 and in 19 out of 21 cases in clinically diagnosed probable PSP. Hence, the study by Massey LA et al., showed that midbrain diameter of less than 9.35 was 100% specific and 83% sensitive for PSP when compared to PD and MSA. Present study also showed similar results with a mean midbrain diameter of 7.8 mm.
Study by Warmuth Metz M et al., compared anteroposterior diameter of suprapontine midbrain of PSP patients with other degenerative parkinsonism [15]. PSP had significantly lower midbrain diameters (13.4 mm) than MSA (16.7 mm) and PD (18.5 mm). PSP patients had anteroposterior midbrain diameter smaller than PD patients (13.5 vs 15.5 mm). Righini A et al., also demonstrated statistically significant similar results of lower midbrain diameter of 13.2 mm vs 16.5 in PDV [16].
In PSP, mean midbrain-to-pons ratio in present study was 0.45 with a SD of 0.03. When compared to other groups midbrain pons ratio in PSP was significantly smaller and was statistically significant (p<0.001). In controls, the midbrain constituted two-third of pontine base, whereas in PSP the ratio was less than 52%. In the study, by Massey LA et al., the mean midbrain-to-pons ratio was 0.47 in pathologically proven cases of PSP [14]. In the clinically, diagnosed cases of PSP mean midbrain-to-pons ratio was 0.44. Using a threshold midbrain-to-pons ratio of 0.52 was found to be 100% sensitive and 85.7% specific in diagnosing PSP with 100% positive predictive value [14]. Zanigni S et el., demonstrated midbrain area as most accurate diagnostic marker for differentiating PSP from PD (mean±SD: 75.87±16.95 mm2 vs 132.45±20.94 mm2, respectively; p<0.001) [17].
Limitation(s)
Main limitations of the present study were small cohort, pathologically unconfirmed cases and lack of exact disease duration.
Conclusion(s)
Midbrain diameter and midbrain pons ratio was significantly lower in PSP compared to other parkinson plus syndromes. So, it can be a useful tool for differentiating PSP from other degenerative parkinsonism like MSA and PD.
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration; SD: Standard deviation
PSP: Progressive supranuclear palsy; MSP: Multiple system atrophy; PD: Parkinson’s disease; CBD: Corticobasal degeneration