UTIs are emerging as treatment challenges for the clinicians. The most common uropathogens are now harbouring Multiple Drug Resistance (MDR) mechanisms against the commonly used oral antimicrobial agents for UTI caused by Gram-negative organisms, i.e., Nitrofurantoin, trimethoprim-sulfamethoxazole, fluoroquinolones and second and third-generation cephalosporins [1]. The increase in resistance of gram negative organisms to most of these antibiotics makes outpatient oral therapy a challenge. The therapy becomes difficult with the overuse and misuse of these drugs, particularly in developing countries like India where antibiotics are freely available over the counter.
Increased emergence of ESBL and CRE in pathogens causing UTI further makes the treatment difficult. Carbapenems remain the drug of choice in infections caused by ESBL Enterobacteriaceae; hence their consumption is increasing, which further adds to the selection and spread of carbapenem resistance in these microorganisms [2].
In the light of these emerging MDR organisms, there is an urgent need to re-evaluate old antibiotics. The evaluation of antimicrobials that were not much in clinical use, may offer some ray of hope. Fosfomycin, a phosphonic acid derivative and also known as phosphomycin or phosphonomycin, seems to be one such old antimicrobial offering a ray of hope in treating MDR uropathogens. After a single oral dose of 3g Fosfomycin, its’ peak concentration in urine is achieved within four hours. Thereafter it’s therapeutic levels in urine are maintained upto three days which is sufficient to inhibit most uropathogens [3]. In the Indian scenario, limited data is available regarding clinical use of Fosfomycin for treating UTIs caused by various MDR pathogens despite five decades of Fosfomycin use [4]. The current study was therefore undertaken with the purpose to have an insight into current trends of the uropathogens causing UTI, their antibiotic sensitivity patterns and to evaluate the fosfomycin activity against E.coli and K.pneumoniae, ESBL producers as well as CRE.
Materials and Methods
A retrospective, laboratory-based, study was conducted in the Department of Microbiology in Rajiv Gandhi Super Speciality Hospital, Delhi, India, from December 2018 to November 2019. All the urine samples obtained from clinically suspected UTIs prior to any antibiotic treatment were included in this study. The samples were excluded from the study if the samples were obtained from patients with ongoing antibiotic therapy and also if the samples were repeat samples of the same patient. Confidentiality of the patients was ensured. The demographic data and microbiological analysis results was retrieved from the laboratory register.
Only a single urine sample from each patient was used in the study. The urine sample was received in a sterile, screw-capped and wide mouthed container. Semi quantitative culture of sample was done within two hours of receipt. Using a 4 mm calibrated nichrome loop, a 0.001 mL loopful of urine was inoculated on Cystine-Lactose Electrolyte Deficient (CLED) agar. The inoculated plates were then aerobically incubated for 18-24 hours at 37°C. Growth on CLED was assessed for significant bacteriuria with colony-forming units ≥105/mL of pure growth of single isolates [5]. Vitek-2 Compact (BioMerieux, France) was employed for identification of the isolates and their Antimicrobial Susceptibility Testing (AST). The ESBL and CRE isolates were verified by the CLSI 2019 guidelines using the Advanced Expert System of VITEK-2 automated system; based on analysis of MIC patterns [6].
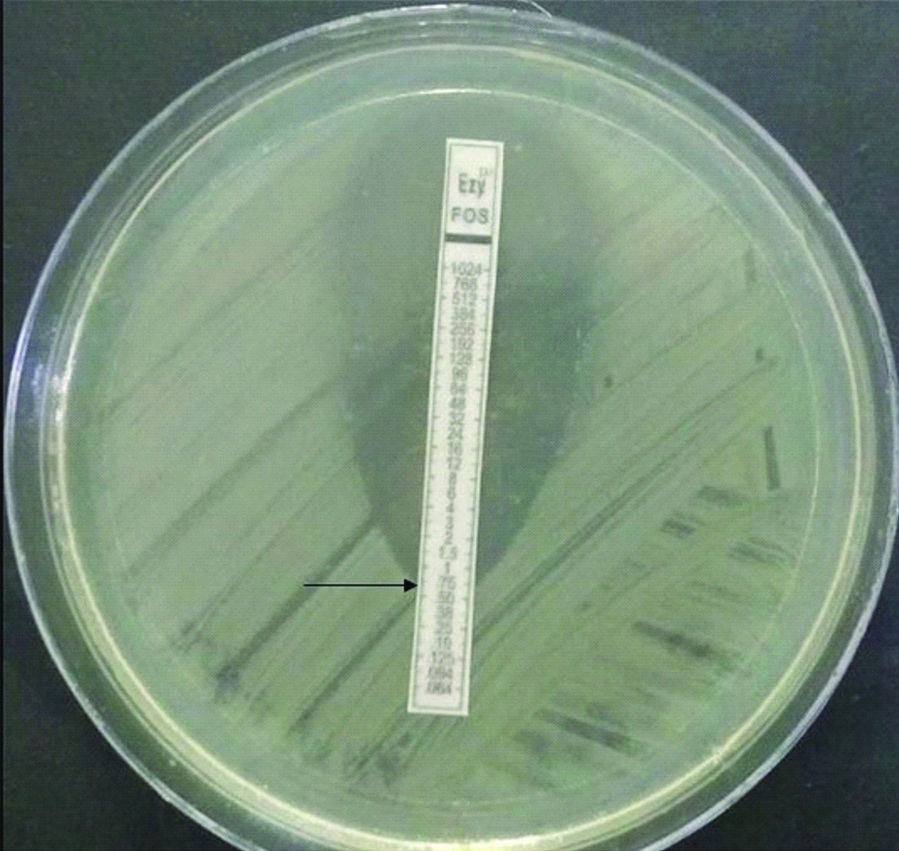
MIC of Fosfomycin was tested by E-test (Biomerieux, India) (as shown in [Table/Fig-1]) with fosfomycin gradient concentrations ranging from 0.064 to 1024 μg/mL, supplemented with 50 μg/mL glucose-6-phosphate. MIC of ≤64 μg was considered sensitive, 128 μg as intermediate and ≥256 μg as resistant to Fosfomycin as per the CLSI 2019 guidelines for E. coli [7]. As the interpretive criteria according to CLSI for Fosfomycin is available only for E. coli and not for other Enterobacteriaceae, the results were interpreted as per CLSI criteria given for E. coli, which have been reported previously in other studies too [8,9].
Figure depicting Fosfomycin E-test on Mueller Hinton Agar showing MIC ≤0.75 μg/Ml (arrow), reported as 1 μg/mL.
Statistical Analysis
Data collected was compiled and entered into Microsoft Excel sheets, doubly checked for any keyboard error and percentages were used to interpret and analyse the findings.
Results
A total of 4000 urine samples were received in the Department of Microbiology for culture and antibiotic sensitivity. Of 4000 urine samples, 226 (5.65%) were culture positive. Out of 226 culture-positive samples, 112 (49.5%) isolates were ESBL and CRE Enterobacteriaceae. Most of the patients were males 64/112 (57.14%), and 48/112 (42.86%) were females. Of the 112 patients, the mean age of male and female patients was 52.81±2.1 years and 44.8±2.18 years, respectively.
Out of 112 isolates, a total of 96 isolates were ESBL-producing Enterobacteriaceae, consisting of 88 isolates of ESBL E.coli and eight isolates of ESBL K. pneumonia and a total of 16 isolates were CRE, consisting of 14 isolates of CRE-E.coli and two isolates of CRE Klebsiella species.
The sensitivity pattern to various antibiotics seen in ESBL positive Enterobacteriaceae and CRE is shown in [Table/Fig-2]. Fosfomycin was found to be sensitive in 88/96 (91.67%) ESBL positive Enterobacteriaceae while it was 14/16 (87.5%) sensitive in CRE. Antimicrobials such as Ampicillin (100%), cefixime (95.83%), ciprofloxacin (91.67%), cotrimoxazole (68.75%) and amoxicillin-clavulanic acid (66.67%) showed a high percentage of resistance rates. Lower rates of resistance were seen in ertapenem (4.17%), amikacin (8.33%), piperacillin-tazobactam (27.09%) and Nitrofurantoin (29.17%).
Antibiotic susceptibility pattern in ESBL and CR- Enterobacteriaceae.
| Antibiotics | ESBL enterobacteriaceae (n=96) | Carbapenem resistant enterobacteriaceae (n=16) |
|---|
| Sensitivity (%) | Resistant (%) | Sensitivity (%) | Resistant (%) |
|---|
| Ampicillin | 0 (0%) | 96 (100%) | 0 (0%) | 16 (100%) |
| Amoxycillin clavulanic acid | 32 (33.33%) | 64 (66.67%) | 0 (0%) | 16 (100%) |
| Piperacillin tazobactam | 70 (72.91%) | 26 (27.09%) | 0 (0%) | 16 (100%) |
| Cefixime | 4 (4.17%) | 92 (95.83%) | 0 (0%) | 16 (100%) |
| Ceftriaxone | 2 (2.08%) | 94 (97.92%) | 0 (0%) | 16 (100%) |
| Ceftazidime | 18 (18.75%) | 78 (81.25%) | 0 (0%) | 16 (100%) |
| Ertapenem | 92 (95.83%) | 4 (4.17%) | 0 (0%) | 16 (100%) |
| Amikacin | 88 (91.67%) | 8 (8.33%) | 4 (25%) | 12 (75%) |
| Gentamycin | 56 (58.33%) | 40 (41.67%) | 4 (25%) | 12 (75%) |
| Ciprofloxacin | 8 (8.33%) | 88 (91.67%) | 0 (0%) | 16 (100%) |
| Cotrimoxazole | 30 (31.25%) | 66 (68.75%) | 0 (0%) | 16 (100%) |
| Nitrofurantoin | 68 (70.83%) | 28 (29.17%) | 8 (50%) | 8 (50%) |
| Fosfomycin | 88 (91.67%) | 8 (8.33%) | 14 (87.5%) | 2 (12.5%) |
[Table/Fig-3] depicts the sensitivity pattern of various antibiotics to ESBL positive E.coli; the most common uropathogen isolated. These isolates were most sensitive to Fosfomycin (97.73%) and carbapenems while resistance was observed for cephalosporins, aminoglycoside and fluoroquinolone group of antimicrobials.
Antimicrobial susceptibility pattern in ESBL E.coli isolates.
| Antibiotics | ESBL* E.coli (n=88) |
|---|
| Sensitivity (%) | Resistant (%) |
|---|
| Ampicillin | 0 (0%) | 88 (100%) |
| Amoxycillin clavulanic acid | 30 (34.09%) | 58 (65.91%) |
| Piperacillin tazobactam | 64 (72.73%) | 24 (27.27%) |
| Cefixime | 4 (4.55%) | 84 (95.45%) |
| Ceftriaxone | 2 (2.27%) | 86 (97.73%) |
| Ceftazidime | 18 (20.45%) | 70 (79.55%) |
| Ertapenem | 86 (97.73%) | 2 (2.27%) |
| Amikacin | 82 (93.18%) | 6 (6.82%) |
| Gentamycin | 36 (40.91%) | 52 (59.09%) |
| Ciprofloxacin | 4 (4.55%) | 84 (95.45%) |
| Cotrimoxazole | 28 (31.82%) | 60 (68.18%) |
| Nitrofurantoin | 68 (77.27%) | 20 (22.73%) |
| Fosfomycin | 86 (97.73%) | 2 (2.27%) |
*ESBL: Extended-spectrum beta-lactamase
Fosfomycin susceptibility was seen in 88/96 (91.67%) of ESBL positive isolates which included 86/88 (97.73%) of ESBL positive E.coli and to a lesser extent in ESBL positive Klebsiella species 2/8 (25%) [Table/Fig-4,5]. Fosfomycin susceptibility among CRE isolates was also high [Table/Fig-4] in the study, at 87.5% (14/16) out of which 100% (14/14) Carbapenem-Resistant (CR) E.coli isolates were Fosfomycin susceptible whereas two isolates of CR Klebsiella spp. were resistant [Table/Fig-5].
Susceptibility of fosfomycin to the multidrg resistant uropathogens isolated.
| Group | Fosfomycin susceptible (%) | Fosfomycin resistant (%) |
|---|
| ESBL* (n=96) | 88 (91.67%) | 8 (8.33%) |
| CRE** (n=16) | 14 (87.5%) | 2 (12.5%) |
ESBL*: Extended-spectrum beta-lactamase, CRE**: Carbapenem-resistant enterobacteriaceae
Fosfomycin sensitivity of ESBL and CRE E.coli and Klebsiella spp isolates.
| Fosfomycin | E.coli | Klebsiella spp |
|---|
| ESBL* | CRE** | ESBL* | CRE** |
|---|
| Sensitive | 86/88 (97.73%) | 14/14 (100%) | 2/8 (25%) | 0/2 (0%) |
| Resistant | 2/88 (2.27%) | 0/14 (0%) | 6/8 (75%) | 2/2 (100%) |
| Total | 88 | 14 | 8 | 2 |
ESBL*: Extended-spectrum beta-lactamase, CRE**: Carbapenem-resistant enterobacteriaceae
Discussion
The increasing trends of ESBL and CRE among Enterobacteriaceae isolate both from the community and health care settings are creating havoc. ESBL and CRE belong to the “Critical” Priority pathogen list by WHO which are resistant to the best available antibiotics like carbapenems and 3rd generation cephalosporins for treating MDR bacteria [10]. There is an urgent need for a new drug or to review an old existing one that is orally active with low existing resistance to combat the present situation.
An old broad-spectrum bactericidal agent, Fosfomycin acts by disrupting bacterial cell-wall synthesis [11]. It has good invitro activity against the common uropathogens causing UTI, particularly towards the Enterobacteriaceae [12]. The use of Fosfomycin is prevalent for UTI caused by E.coli, the most common uropathogens [11]. Recent studies have showed encouraging Fosfomycin invitro activity against MDR Gram-negative pathogens [13,14]. In cases of uncomplicated UTI, a reliable treatment modality is use of fosfomycin tromethamine, according to a study by Schito GC because of its advantages (single oral dose and a sustained high urinary concentration) that kills bacteria rapidly and opportunity for mutant selection will decrease subsequently. This drug is not present in animal feed; resistance is mostly acquired by a chromosomal mutation which does not spread easily. Also, fosfomycin tromethamine has excellent tolerability and safety [15]. Along with low resistance rates, the other benefits of fosfomycin include less cost, dosage friendly, non-toxic, non-allergic and little tendency to display cross-resistance to other antibiotics [16].
In the present study, authors evaluated the invitro activity of fosfomycin because of its unique properties such as the broad spectrum of activity against gram-negative organisms and oral availability in a single dose formulation which is an essential factor in treating UTIs on an outpatient basis.
In the present retrospective study, a total of 4000 non-repetitive urine samples obtained from patients diagnosed with clinical suspicion of UTI were assessed. The invitro activity of fosfomycin with other antimicrobials in ESBL positive and CR E.coli and Klebsiella species were evaluated. Amongst culture positive samples for significant bacteriuria, 96/226 (42.48%) isolates were found to be ESBL positive uropathogens which was within the range of various other studies which reported the prevalence of ESBL positive uropathogens in UTI to range from 21.8% to 64.8% [4,12,17].
On assessing the AST pattern, high antimicrobial resistance was observed amongst ESBL and CRE uropathogens for fluroquinolones, ampicillin and cefixime [Table/Fig-2]. Sastry S et al., and Patwardhan V and Singh S also reported similar rates of high antimicrobial resistance in two different studies [18,19]. Patwardhan V and Singh S in a study from North India, reported lower invitro activity of ampicillin, amoxicillin-clavulanic acid, cotrimoxazole, nitrofurantoin and norfloxacin [19]. ESBL producing Enterobacteriaceae isolates were also susceptible to beta-lactam/beta-lactam inhibitor combinations like piperacillin-tazobactam (72.91%), aminoglycosides, e.g., Amikacin (91.67%) and Carbapenem like Ertapenem (95.83%) [Table/Fig-2]. Using these parenteral drugs for the treatment of UTI will further lead to an increase in the hospitalisation rate.
The high sensitivity of fosfomycin in ESBL positive E.coli observed in the study was in accordance with the findings of other recent studies done by Sabharwal ER and Sharma R, (95%) and Patwardhan V and Singh S, (96.5%) [4,19]. High fosfomycin susceptibility in CRE isolates at 87.5% with 100% fosfomycin susceptibility amongst CR E.coli have been presented by other contemporary studies around the world [12,20,21]. High fosfomycin susceptibility among CRE observed in the study further gives hope in treating CRE causing UTI, rather than using other nephrotoxic drugs which remain the only available option for treating such cases. In the present study, 91.67% (88/96) ESBL producing Enterobacteriaceae (E.coli and Klebsiella spp.) isolates and 87.5% (14/16) of CRE isolates [Table/Fig-4] were susceptible to fosfomycin which was similar to the findings of a study done by Patel B et al., in which 92% and 72.34% of ESBL positive and CRE isolates were respectively fosfomycin sensitive [22].
In the outpatient department, where oral antibiotics are preferred, minimal options are available for the oral treatment for UTI. In this study, the only available oral antibiotic with good sensitivity in ESBL positive strains other than Fosfomycin was Nitrofurantoin (70.83%). Amongst other drugs available in oral formulations, the combination antibiotic such as amoxicillin-clavulanic acid showed a high percentage of resistance in both ESBL (66.67%) positive and CRE (100%) strains. Quinolones like ciprofloxacin also displayed a high percentage of resistance (91.67%) in ESBL positive isolates.
The most frequently isolated Gram-negative uropathogens encountered in the present study was ESBL producing E.coli, which was found to be highly susceptible to 97.73% (86/88) to fosfomycin. Similar findings were reported by Banerjee S et al., who found that fosfomycin was sensitive in 134/137 (97.81%) ESBL producing E.coli [12].
In the era of global antimicrobial resistance, resistance to fosfomycin is observed but not at the same pace as compared to the rest of antimicrobial classes despite its usage since the 1970s [23,24]. Many studies in the last decade have noted a range of fosfomycin resistance rates varying from 0-49% amongst MDR uropathogens [Table/Fig-6] [4,6,9,12,15,17,19-22,24-29]. In the present study, resistance to fosfomycin in ESBL-producing and CR- Enterobacteriaceae was noted to be 8.33% and 12.5%, respectively [Table/Fig-4]. Although low resistance rate was observed, continuous monitoring of fosfomycin susceptibility is warranted to keep a check on any increase in resistance pattern and further to aid in its clinical application.
Studies showing invitro susceptibility pattern of fosfomycin amongst isolated uropathogens [4,6,9,12,15,17,19-22,24-29].
| Study, year of publication, country, [Ref No.] | Uropathogens isolated | Susceptible to Fosfomycin (%) | Resistance to Fosfomycin (%) | Method of testing |
|---|
| Schito GC, 2003, Italy, [15] | Escherichia coli | 99 | 1 | Disk diffusion method |
| Maraki S et al., 2009, Greece, [9] | Enterobacter species | 75 | 25 | Disk diffusion method |
| Klebsiella pneumonia | 82.3 | 17.7 |
| Proteus mirabilis | 96.7 | 3.3 |
| Escherichia coli | 100 | 0 |
| Liu H et al., 2011, Taiwan, [24] | ESBL*-producing Klebsiella | 57.6 | 42.4 | Disk diffusion method |
| ESBL*-producing E. coli | 95.5 | 4.5 |
| Muvunyi CM et al., 2013, Rwanda, [25] | Escherichia coli | 99 | 1 | Disk diffusion method |
| Pogue JM et al., 2013, USA, [21] | CR**-Klebsiella | 57 | 43 | E-test |
| CR**-Enterobacter | 80 | 20 |
| CR**-E. coli | 100 | 0 |
| Gupta V et al., 2013, India, [26] | ESBL*-producing E. coli | 100 | 0 | E-test, Disk diffusion method |
| Lai B et al., 2014, China, [27] | ESBL*-producing E. coli | 90 | 10 | Disk diffusion method |
| Sultan A et al., 2015, India, [17] | ESBL*-producing Enterobacteriaceae | 100 | 0 | Disc diffusion method |
| Sabharwal ER and Sharma R, 2015, India, [4] | ESBL*-producing E. coli | 95 | 5 | Disc diffusion method |
| Sardar A et al., 2017, India, [28] | Escherichia coli | 100 | 0 | Disc diffusion method |
| Banerjee S et al., 2017, India, [12] | CR**-E. coli | 87.5 | 12.5 | E-test |
| CR**-Klebsiella | 90.47 | 9.53 |
| ESBL*-producing Klebsiella | 93.61 | 6.39 |
| ESBL*-producing E. coli | 97.81 | 2.19 |
| Patel B et al, 2017, India, [22] | CR**-producing Enterobacteriaceae | 72.34 | 27.66 | E-test |
| ESBL*-producing Enterobacteriaceae | 92 | 8 |
| Patwardhan V and Singh S, 2017, India, [19] | ESBL*-producing Klebsiella | 92.1 | 7.9 | Disc diffusion method |
| ESBL*-producing E. coli | 97.8 | 2.2 |
| Sahu M et al., 2017, India, [6] | ESBL* only | 67 | 33 | E-test |
| CRE***+ESBL* | 42 | 49 |
| Dalai S et al., 2018, India, [29] | CR**-E. coli | 90.9 | 9.1 | Automated microbroth dilution test |
| CR**-Klebsiella | 87.5 | 12.5 |
| ESBL*-producing Klebsiella | 96.8 | 3.2 |
| ESBL*-producing E. coli | 87.1 | 12.9 |
| Amladi AU et al., 2019, India, [20] | CR**-Klebsiella | 94 | 6 | E-test |
| CR**-E. coli | 98.8 | 1.2 |
| Present study, 2020, India | CR**-E. coli | 100 | 0 | E-test |
| CR**-Klebsiella | 0 | 100 |
| ESBL*-producing Klebsiella | 25 | 75 |
| ESBL*-producing E. coli | 97.73 | 2.27 |
*ESBL: Extended-spectrum beta-lactamase; CR**-Carbapenem-resistant; CRE***-CR-producing enterobacteriaceae
Limitation(s)
This was a retrospective study in which only invitro susceptibility of fosfomycin was evaluated. In vivo/clinical efficacy of the fosfomycin could not be evaluated in this study.
Conclusion(s)
The emergence of increasing drug-resistant isolates of ESBL producing Enterobacteriaceae and CRE (E.coli and Klebsiella species) to commonly used antibiotics like fluoroquinolones, cephalosporins and other β-lactams was observed in the present study. This trend of rise in isolation of MDR uropathogens poses a challenge to the current armamentarium for the treatment of UTIs. In light of the above findings, fosfomycin shows promising results as a re-emerging antibiotic for the treatment of UTI because of its unique mechanism of action, low incidence of resistance, oral availability with single-dose administration and less propensity to display cross-resistance to other antibiotics. With the unavailability of newer antibiotics and with only a few alternative drugs available for these resistant pathogens, it necessitates revival of the use of old antibiotics like Fosfomycin.
*ESBL: Extended-spectrum beta-lactamase
ESBL*: Extended-spectrum beta-lactamase, CRE**: Carbapenem-resistant enterobacteriaceae
ESBL*: Extended-spectrum beta-lactamase, CRE**: Carbapenem-resistant enterobacteriaceae
*ESBL: Extended-spectrum beta-lactamase; CR**-Carbapenem-resistant; CRE***-CR-producing enterobacteriaceae