Corona Virus Disease of 2019 (COVID-19) is spreading throughout the world and the United States with confirmed cases in all 50 states. Cytokine Release Syndrome (CRS), or cytokine storm, is being increasingly reported with severe cases of COVID-19 patients and is a common cause of death in these patients. Hereby, authors report a case of critically ill COVID-19 patient who developed cytokine storm. She had a remarkable increase in inflammatory markers and went into multiorgan failure and death in less than 48 hours. Among inflammatory markers, ferritin has a high sensitivity for CRS. This case report sheds light on the importance of following the level of inflammatory markers (especially ferritin) closely in COVID-19 patients. The goal is to diagnose CRS before the patient goes into a full cytokine storm and multiorgan failure, as it may be too late to react then. Even when the patient is clinically stable, a high ferritin level could be the calm before the storm.
Cytokine release syndrome, Haemophagocytic lymphohistiocytosis, Macrophage activation syndrome
Case Report
A 66-year-old Caucasian female presented to the emergency room on 3/17/2020 with shortness of breath for 3 days duration. She was a former smoker (with a 34-pack/year smoking history), and had a medical history of hypertension, obstructive sleep apnea, fibromyalgia, and gastrointestinal reflux disease. The patient had been complaining of fever, non-productive cough, and nasal discharge for 3 days. She did not have any recent travel or sick contact history.
Upon arrival to the emergency room, the patient was found to be in acute respiratory distress with a respiratory rate of 35 breaths per minute, hypotensive with a BP of 55/16 mmHg and febrile with a Temperature of 101.5°F (38.6°C). She was intubated, started on broad spectrum antibiotics (vancomycin and cefepime intravenously) and vasopressors (norepinephrine infusion).
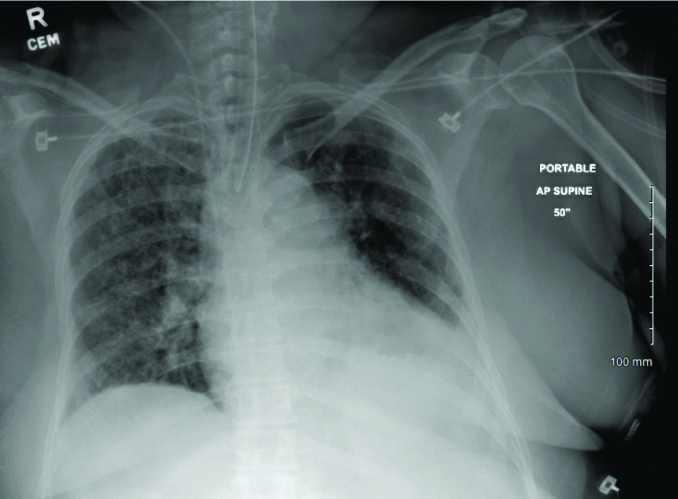
Her chest X-ray showed bilateral multifocal infiltrates [Table/Fig-1]. Viral pneumonia was high in the differential diagnosis, but bacterial pneumonia with superimposed pulmonary oedema and acute interstitial pneumonia were also considered. Nasopharyngeal swab for respiratory viral panel (qualitative DNA PCR test) was negative for Influenza A, B and Respiratory Syncytial Virus. A nasopharyngeal swab for COVID-19 virus (qualitative RNA PCR test) was sent and the patient was placed on airborne and contact isolation in the ICU. Infectious disease specialist was consulted, and the patient was started on hydroxychloroquine and azithromycin.
Chest X-ray on admission: Endotracheal tube and right IJ central venous catheter seem to be in satisfactory positions. Patchy airspace disease throughout both lung fields suggesting multifocal bilateral pneumonia.
The COVID-19 test came back positive. Troponin level was also found to be elevated on presentation; however, serial troponin levels trended down (hs-c Troponin T was 258 ng/L on presentation and then, it was 177 ng/L and 147 ng/L when measured 8 and 16 hours later) and EKG on presentation showed no ischaemic changes. Blood, sputum, and urine cultures showed no bacterial growth. Urine legionella antigen was also negative and there was no other clear source of infection.
The patient improved haemodynamically and did not require vasopressor support between day 3 and 10 of admission. She remained intubated with mild improvement in the ventilator setting (FiO2 of 60-80% with PEEP of 10-14 cm H2O). The patient continued to experience intermittent fever, up to 102.9°F (39.4°C), and the chest X-ray showed no improvement in the bilateral multifocal infiltrates.
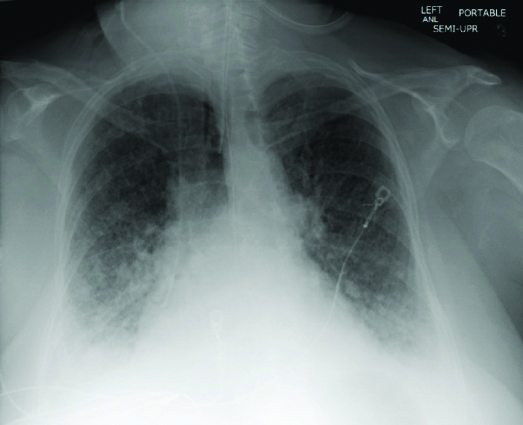
On admission day 10, the patient became hypotensive again requiring vasopressor support (norepinephrine infusion). Her respiratory status also worsened (requiring 100% FiO2 with a PEEP of 15 cm H2O). Repeat chest X-ray showed worsening diffuse airspace disease [Table/Fig-2]. The patient also developed acute kidney injury (serum creatinine was 1.44 mg/dL) with hyperkalemia (potassium was 5.9 mmol/L). There was also a concern that volume overload was contributing to the worsening respiratory status (NT Pro-BNP was 4,205 pg/mL). At this point, nephrology specialist was consulted, and the patient was started on renal replacement therapy after informed consent was obtained from the family. A temporary dialysis catheter was placed in the right femoral vein and Continuous Renal Replacement Therapy (CRRT), in the form of Continuous Venovenous Haemodiafiltration (CVVHDF), was initiated.
Chest X-ray on day 10 of admission: Endotracheal tube and right IJ central venous catheter are unchanged. Enteric tube extends into the upper abdomen. There are ill-defined interstitial opacities in both lung fields with associated septal thickening. This is suggestive of ARDS. Airspace opacities are more confluent in the bases.
Given the clinical deterioration on day 10, serum ferritin level was measured, and was severely elevated from 31,721 ng/mL on day 10, up from 656 ng/mL on day 8. It was not measured on day 9. The high ferritin level along with other high inflammatory markers [Table/Fig-3] was suggestive of CRS, aka Cytokine Storm. Remdesivir was delivered to the hospital on the 10th night of admission (this was approved based on compassionate use outside of clinical trials). The plan was to start Remdesivir to target the virus directly as well as plasma exchange to target the CRS.
Laboratory values on day 10 of admission.
| Name of blood test | Value (normal range) |
|---|
| Ferritin | 31,721 (13-150 ng/mL) |
| CRP | 193.27 (<5.00 mg/L) |
| Fibrinogen | 474 (196-447 mg/dL) |
| LDH | >25000 (135-214 U/L) |
| ALT | 2,230 (<41 U/L) |
| AST | >7,000 (<41 U/L) |
| Triglyceride | 342 (<150 mg/dL) |
CRP: C-reactive protein; LDH: Lactate dehydrogenase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase
On the morning of day 11 of admission, the patient became more haemodynamically unstable with increased vasopressors requirements. Oxygen requirement improved after 16 hours of prone ventilation (FiO2 improved from 100% to 75% with a 15 cm H2O of PEEP). The patient underwent CRRT with no reported side effects or machine-related clotting. Clinicians were able to remove more than 5000 cc of fluids via CRRT. Metabolic panel showed improved hyperkalemia (with a potassium of 5.1 mmol/L). A few short runs of ventricular tachycardias were noted on the heart monitor. This was followed by sustained ventricular tachycardia and cardiac arrest, so ACLS protocol was activated. Unfortunately, the patient did not respond to resuscitation and died on the 11th day of admission at 12:03 pm.
Discussion
COVID-19 is rapidly spreading throughout the world. As of April 7, 2020, there were a total of 395,926 COVID-19 cases reported in the United States, including 12,757 deaths [1]. CRS or also known as Cytokine Storm, macrophage activation syndrome or Haemophagocytic Lymphohistiocytosis (HLH), is a state of hyperinflammation that is caused by a sharp increase in pro-inflammatory cytokines, like TNF-α, IL-1β, IL-8, and IL-6 and can lead to multi organ failure and death [2]. CRS can be associated with immune system related diseases or therapies, such as CAR-T cell therapy, sepsis in organ transplantation and viral infections [3]. In adults, the most common cause of CRS is viral infections [4]. It has also been reported in 3.7-4.3% of sepsis cases [5].
CRS is being increasingly recognised in severe cases of COVID-19 leading to multiorgan failure and death [3,6,7]. CRS is a clinical diagnosis and common clinical features include: unremitting fever, cytopenias, hyperferritinemia and pulmonary symptoms including Acute Respiratory Distress Syndrome (ARDS) [6]. Fardet L et al., developed and validated the H score criteria to predict the probability of the presence of secondary HLH [8]. However, prompt treatment should not be delayed until the fulfilment of all the diagnostic criteria given the high mortality and rapid deterioration of CRS. This is more so the case in the COVID-19 pandemic with the limited resources in healthcare system in comparison to the rising number of COVID-19 patients. Furthermore, given the shortage of personal protective equipment and the risk of exposing healthcare personnel to the virus, one should be very judicious in ordering only the necessary laboratory and imaging tests. Serum ferritin level is one of the most sensitive inflammatory markers for CRS [9,10]. Serum ferritin level >2000 ng/mL was found to have a 70% sensitivity and 68% specificity for CRS diagnosis in non COVID-19 patients [11].
The current case report and our observations in other COVID-19 patients have changed our clinical practice. It is suggested that serum ferritin level should be checked in all COVID-19 patients on admission, as a baseline. Then serum ferritin level should be followed, during the hospital stay, with any clinical deterioration or lack of improvement. We suggest a level >1000 ng/mL, or doubling of the level within 24-48 hours, should raise high clinical suspicion for CRS. In a recent retrospective study of 150 COVID-19 patients, one of the predictors of high mortality was elevated ferritin level (mean 1297·6 ng/mL in nonsurvivors vs 614·0 ng/mL in survivors; p<0·001) [12].
Chest X-ray in this patient showed an ARDS like appearance. However, it is important to make the distinction between COVID-19 severe respiratory distress and the typical ARDS. COVID-19 severe respiratory distress can be associated with preserved lung mechanics despite severe hypoxemia. It is also associated with high respiratory compliance and shunt fraction with the need for prolonged mechanical ventilation [13]. When lung tissues of severe COVID-19 cases were examined, typical features of ARDS like diffuse alveolar damage with hyaline membrane, inflammation, and Type II pneumocyte hyperplasia were not found. Instead, deposition of terminal complement components C5-9 (known as membrane attack complex), C4d and Mannose Binding Lectin (MBL)-associated serine protease was found in the microvasculature. This suggests a catastrophic microvascular injury mediated by the activation of complement pathways (both the alternative and lectin-based pathway) and procoagulant state [14].
Conclusion(s)
CRS can happen in severe cases of COVID-19 patients and can be fatal. The key is early diagnosis and treatment. Perhaps, with routine measurement of serum ferritin level in COVID-19 patients, early cases of CRS can be detected and treated, which could lead to a decrease in mortality. With such a novel viral infection taking many lives away every day, we ought to pay close attention to details and be vigilant in our fight against it.
CRP: C-reactive protein; LDH: Lactate dehydrogenase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 05, 2020
Manual Googling: May 07, 2020
iThenticate Software: Jun 13, 2020 (3%)
[1]. Bialek S, Bowen V, Chow N, Curns A, Gierke R, Hall A, Geographic Differences in COVID-19 Cases, Deaths, and Incidence-United States, February 12-April 7, 2020MMWR Morb Mortal Wkly Rep [Internet] 2020 Apr 17 [cited 2020 Apr 19] 69(15):465-71.Available from: http://www.cdc.gov/mmwr/volumes/69/wr/mm6915e4.htm?s_cid=mm6915e4_w10.15585/mmwr.mm6915e432298250 [Google Scholar] [CrossRef] [PubMed]
[2]. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG, Into the eye of the cytokine stormMicrobiol Mol Biol Rev 2012 76(1):16-32.10.1128/MMBR.05015-1122390970 [Google Scholar] [CrossRef] [PubMed]
[3]. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ, The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortalityInt J Antimicrob Agents 2020 2020:10595410.1016/j.ijantimicag.2020.10595432234467 [Google Scholar] [CrossRef] [PubMed]
[4]. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X, Adult haemophagocytic syndromeIn: The Lancet 2014 Lancet Publishing Group:1503-16.10.1016/S0140-6736(13)61048-X [Google Scholar] [CrossRef]
[5]. Karakike E, Giamarellos-Bourboulis EJ, Macrophage activation-like syndrome: A distinct entity leading to early death in sepsis. Vol. 10Frontiers in Immunology 2019 Frontiers Media SA10.3389/fimmu.2019.0005530766533 [Google Scholar] [CrossRef] [PubMed]
[6]. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, COVID-19: consider cytokine storm syndromes and immunosuppression. Vol. 395The Lancet 2020 Lancet Publishing Group:1033-34.10.1016/S0140-6736(20)30628-0 [Google Scholar] [CrossRef]
[7]. Vaninov N, In the eye of the COVID-19 cytokine stormNat Rev Immunol [Internet] 2020 Apr 6 [cited 2020 Apr 19] 1-1Available from: http://www.ncbi.nlm.nih.gov/pubmed/32249847 [Google Scholar]
[8]. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Development and validation of the hscore, a score for the diagnosis of reactive hemophagocytic syndromeArthritis Rheumatol 2014 66(9):2613-20.10.1002/art.3869024782338 [Google Scholar] [CrossRef] [PubMed]
[9]. Trottestam H, Horne AC, Aricò M, Egeler RM, Filipovich AH, Gadner H, Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: Long-term results of the HLH-94 treatment protocolBlood [Internet] 2011 Oct 27 [cited 2020 Apr 21] 118(17):4577-84.Available from: http://www.ncbi.nlm.nih.gov/pubmed/2190019210.1182/blood-2011-06-35626121900192 [Google Scholar] [CrossRef] [PubMed]
[10]. Allen CE, Yu X, Kozinetz CA, McClain KL, Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosisPediatr Blood Cancer 2008 50(6):1227-35.10.1002/pbc.2142318085676 [Google Scholar] [CrossRef] [PubMed]
[11]. Lehmberg K, Mcclain KL, Janka GE, Allen CE, Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosisPediatr Blood Cancer 2014 61(11):2101-03.10.1002/pbc.2505824753034 [Google Scholar] [CrossRef] [PubMed]
[12]. Ruan Q, Yang K, Wang W, Jiang L, Song J, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, ChinaIntensive Care Medicine 2020 Springer:110.1007/s00134-020-06028-z32253449 [Google Scholar] [CrossRef] [PubMed]
[13]. Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D, Covid-19 does not lead to a “typical” acute respiratory distress syndromeAm J Respir Crit Care Med [Internet] 2020 Mar 30 [cited 2020 May 16] 201(10):1299-300.Available from: https://www.atsjournals.org/doi/10.1164/rccm.202003-0817LE10.1164/rccm.202003-0817LE32228035 [Google Scholar] [CrossRef] [PubMed]
[14]. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five casesTransl Res 2020 10.1016/j.trsl.2020.04.00732299776 [Google Scholar] [CrossRef] [PubMed]