The Oesophagogastroduodenoscopy (EGD) is the most common method suggested for upper gastrointestinal disease patients [1,2]. But, foam and bubbles can accumulate in the stomach and duodenum, reducing endoscopic visibility. Subtle lesions can be missed due to the foam and bubbles. This can decrease diagnostic accuracy and reduces patient comfort as it takes a lot of time clearing the foam and bubbles [3]. Activated dimethicone (polydimethylsiloxane and silicon dioxide or simethicone) has proven to be a good defoaming agent for use as an endoscopic premedication to remove bubbles. Activated dimethicone is not absorbed systemically following oral administration [4]. There are no significant side effects of Activated dimethicone reported till now. Activated dimethicone can be given up to 500 milligrams in a day without any systemic toxicity. Activated dimethicone reduces the surface tension of air bubbles, causing small bubbles to coalesce and collapse to release trapped air, thus reducing the foam and bubbles in the upper gastrointestinal tract [5,6].
This premedication regimen varies among different clinical practices. A recent meta-analysis study analysed 10 high quality studies comparing the efficacy of dimethicone with N-acetyl cysteine or alone intending to provide better basis for choosing the anti-foaming agent and conclusion faced heterogeneity in the methods used and inconsistency in the outcomes or statistical outputs [7]. There is not much literature regarding the efficacy of activated dimethicone in improving mucosal visibility during EGD in Indian population. Therefore, this study was planned to collect and analyse data from two EGD units, one unit using pre-procedure activated dimethicone and the other unit not using any preparation. From this data, we assessed the efficacy of activated dimethicone in improving endoscopic visibility in Indian population. The primary objective of the present study was to assess the Total Mucosal Visibility Score (TMVS) and individual mucosal visibility scores. The secondary objectives included time taken for completion of EGD and amount of water flush used, assessing post-procedural bloating.
Materials and Methods
This case-control study was conducted at a tertiary hospital in Chennai. This study was carried out between February 2017 and February 2018. The patient population included a fair representation from urban and rural areas. It includes people from varied socioeconomic strata. Institutional Ethics Committee approval (CSP-MED/17/JAN/33/26) was obtained for the study. This study included 2917 patients. The sample size was determined as per previous studies [8-10].
Inclusion criteria: All consecutive patients referred for routine EGD with a minimum age of 18 years were included in the study.
Exclusion criteria: Emergency cases, pregnant or breastfeeding women, patients with known stricture or stenosis of upper digestive tract and patients that already received activated dimethicone pre-endoscopy as part of their standard care within past 2 weeks were excluded from the study.
Written informed consent was collected from the study participants before performing EGD. Data was collected from two EGD units. Patients in both units were nil per oral for a period of 8 hours before the procedure. Group S received 125 mg of activated dimethicone (Nodis, Retort Pharmaceuticals, Chennai, India) in 60 mL of water 30 minutes prior to EGD. Patients of group C underwent EGD without any preparation. Patient allocation to each group was based on Out Patient Department Endoscopy Unit registration. Patients registered with unit I were allocated to group S and patients registered with unit II were allocated to Group C. Time taken for completion of study, amount of water flushing, TMVS was recorded for each patient and compared between the two groups.
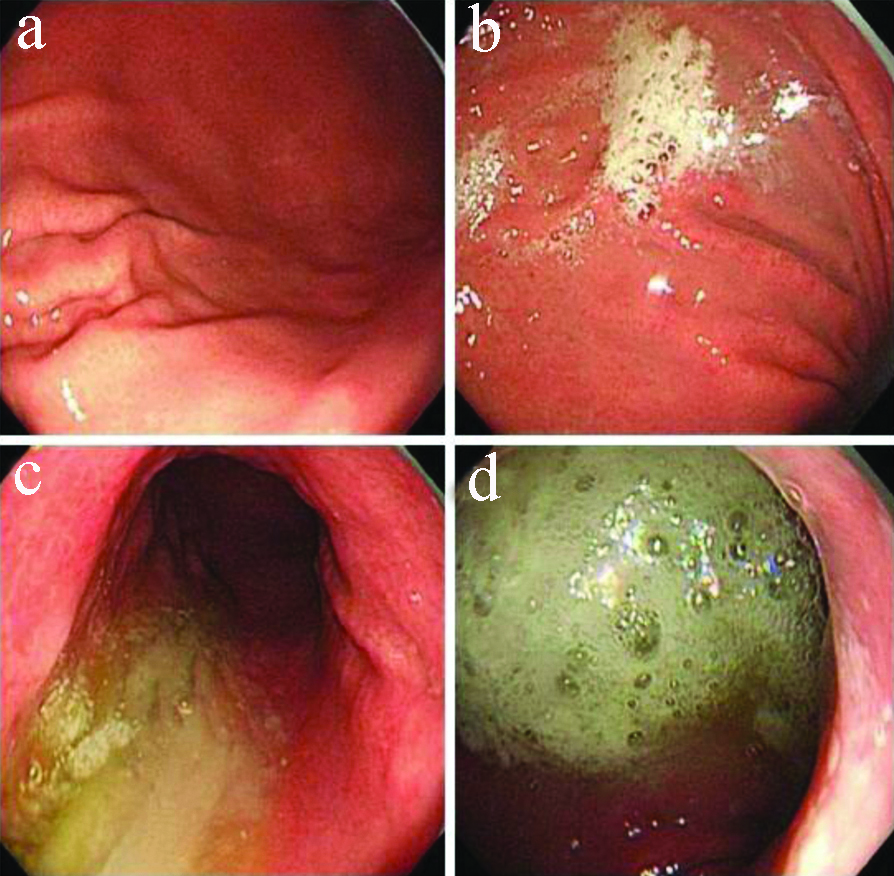
The mucosal visibility scoring was used as described by previous study [6]. Score 1: No adherent mucus; Score 2: Mild mucus not obscuring vision; Score 3: A large amount of mucus obscuring vision and requiring <50 mL water to clear; Score 4: Heavy adherent mucus requiring >50 mL water to clear [Table/Fig-1]. Scoring is done for each area noted in lower oesophagus, stomach (upper body, greater curve), antrum, and duodenum. Total Score ranges from 4 to 16. The Score of 4 indicates best mucosal visibility and 16 indicates poor mucosal visibility.
Scoring of upper gastrointestinal endoscopy mucosal visibility. a) Score 1: No adherent mucus; b) Score 2: Mild mucus not obscuring vision; c) Score 3: A large amount of mucus obscuring vision and requiring <50 mL water to clear; d) Score 4: Heavy adherent mucus requiring >50 mL water to clear.
Statistical Analysis
The collected data were analysed with IBM. SPSS statistics software 23.0 Version. To describe about the data descriptive statistics, frequency analysis, percentage analysis were used for categorical variables and the mean and SD were used for continuous variables. To find the significant difference between the bivariate samples in independent groups, the unpaired sample t-test was used. To find the significance in categorical data chi-square test was used. In both the above statistical tools, the probability value ≤0.05 was considered as significant level.
Results
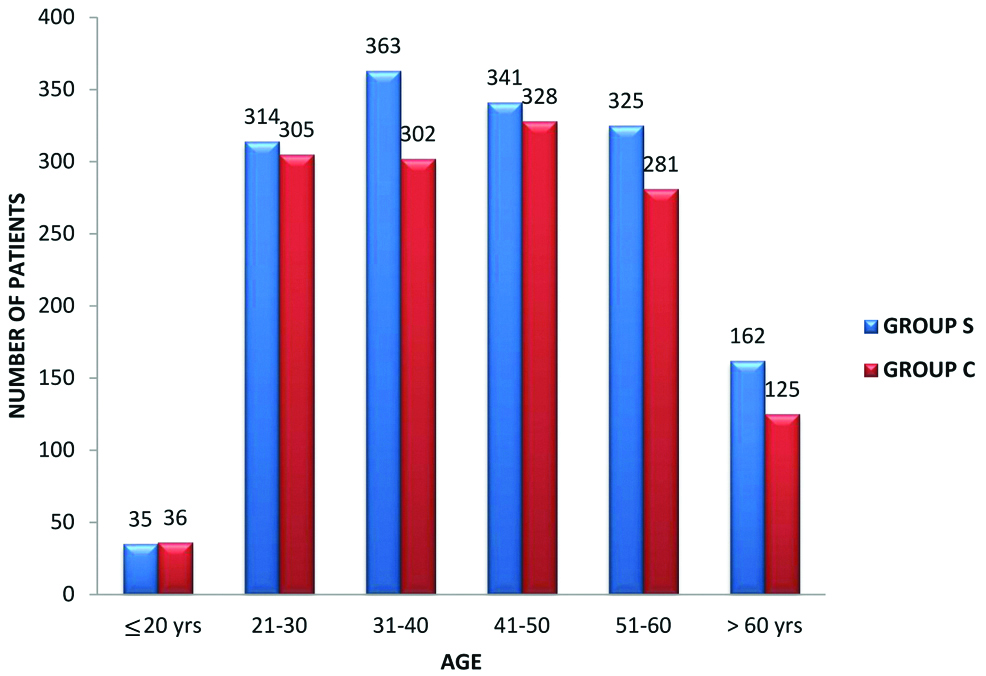
This prospective study included 2917 (1579 men and 1338 women) patients, divided into group S and group C. Group S included 1540 patients and group C included 1377 patients. The demographic details of patients are tabulated in [Table/Fig-2]. The ages Mean±SD of group S and group C were 41±13 and 42±13, respectively. The age wise distribution of patients among the two groups was depicted in [Table/Fig-3]. The maximum number of patient was in age range of 21-60 years in both the groups. The ratios of male to female between group S and group C were 1:0.78 and 1:0.92, respectively. Majority of patients were diagnosed as oesophagitis (27.3%) and 25% were normal [Table/Fig-4]. The means of TMVS between group S and group C were 5.38 and 6.85, respectively. Group S had a significantly (p-value=0.0001) lower TMVS than group C.
Patient demographic characteristics. N-frequency or number of patients. p-value less than 0.05 is considered significant.
| Characteristics | Group S | Group C | p-value |
|---|
| Age (years) (Mean±SD) | 41±13 | 42±13 | 0.04 |
| Sex | Male | 865 | 714 | 0.35 |
| Female | 675 | 663 |
| Indications | N (%) |
| Dyspepsia (N) | | 749 | 664 | 1413 (48.4%) |
| Reflux (N) | | 488 | 426 | 914 (31.3%) |
| Anaemia (N) | | 203 | 156 | 359 (12.3%) |
| Minor UGI bleed (N) | | 25 | 30 | 55 (1.9%) |
| Variceal screening (N) | | 75 | 101 | 176 (6.0%) |
Age wise distribution of patients in study groups.
Patient diagnoses. N-frequency or number of patients, %-Percentage.
| Diagnosis | Group S | Group C |
|---|
| N | % | N | % |
|---|
| Duodenal mucosal erosions | 26 | 1.69% | 25 | 1.82% |
| Duodenal mucosal erythema | 34 | 2.21% | 30 | 2.18% |
| Duodenal mucosal nodularity | 8 | 0.52% | 7 | 0.51% |
| Duodenal ulcer | 66 | 4.29% | 56 | 4.07% |
| Oesophagitis | 420 | 27.27% | 377 | 27.38% |
| Gastric growth | 42 | 2.73% | 27 | 1.96% |
| Gastric mucosal erosions | 127 | 8.25% | 108 | 7.84% |
| Gastric mucosal erythema | 237 | 15.39% | 212 | 15.40% |
| Gastric nodule | 33 | 2.14% | 32 | 2.32% |
| Gastric polyp | 13 | 0.84% | 9 | 0.65% |
| Gastric ulcer | 47 | 3.05% | 40 | 2.90% |
| Large oesophageal varices | 6 | 0.39% | 9 | 0.65% |
| Large oesophageal varices with GOV1 | 4 | 0.26% | 6 | 0.44% |
| Large oesophageal varices with GOV2 | 7 | 0.45% | 9 | 0.65% |
| Large oesophageal varices with severe PHG | 15 | 0.97% | 17 | 1.23% |
| MW tear | 13 | 0.84% | 10 | 0.73% |
| Normal mucosal study | 397 | 25.78% | 342 | 24.84% |
| Small oesophageal varices | 15 | 0.97% | 14 | 1.02% |
| Small oesophageal varices with GOV1 | 5 | 0.32% | 10 | 0.73% |
| Small oesophageal varices with GOV2 | 3 | 0.19% | 9 | 0.65% |
| Small oesophageal varices with Mild PHG | 12 | 0.78% | 13 | 0.94% |
| Small oesophageal varices with severe PHG | 10 | 0.65% | 15 | 1.09% |
GOV 1: Gastricoesophageal Varices extending along the lesser curvature of the stomach; GOV 2: Gastroesophageal Varices extending along the greater curvature towards the gastric fundus; PHG: Portal hypertensive gastropathy; MW: Mallory weiss
The visibility scores of oesophagus, stomach, antrum and duodenum are shown in [Table/Fig-5]. The Mean Mucosal Visibility Scores (MMVS) of group S and group C at oesophagus region are 1.12 and 1.36, respectively. The MMVS of group S and group C at stomach region are 1.77 and 2.12, respectively. The MMVS of group S and group C at gastric antrum region are 1.39 and 1.9, respectively. The MMVS of group S and group C at duodenum region are 1.10 and 1.47, respectively.
Mucosal visibility Scores. p-value # calculated with Pearson Chi-Square test, *calculated with unpaired student’s t-test. MVSE: Mucosal visibility score at oesophagus, MVSS: Mucosal visibility score at stomach; MVSA: Mucosal visibility score at antrum; MVSD: Mucosal visibility score at duodenum; TMVS: Total mucosal visibility score.
| Mucosal visibility Score | 1 | 2 | 3 | 4 | p-value |
|---|
| Oesophagus | Group S | 1367 | 164 | 6 | 3 | 0.0005# |
| Group C | 995 | 292 | 73 | 17 |
| Stomach | Group S | 595 | 745 | 156 | 44 | 0.0005# |
| Group C | 410 | 522 | 309 | 136 |
| Antrum | Group S | 1069 | 365 | 78 | 28 | 0.0005# |
| Group C | 694 | 270 | 273 | 140 |
| Duodenum | Group S | 1396 | 139 | 2 | 3 | 0.0005# |
| Group C | 921 | 291 | 141 | 24 |
| Group | MVSE Mean±SD | MVSS Mean±SD | MVSA Mean±SD | MVSD Mean±SD | TMVS Mean±SD |
| Group S | 1.12±0.35 | 1.77±0.74 | 1.39±0.67 | 1.10±0.32 | 5.38±1.25 |
| Group C | 1.36±0.64 | 2.12±0.95 | 1.9±1.05 | 1.47±0.75 | 6.85±2.08 |
| p-value | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
The mean time taken for completion of procedure was 7.95 minutes and 8.17 minutes for group S and group C, respectively. The time taken was significantly low for group S patients. The mean amount of flush required was significantly low in group S (8.57 mL) than group C (12.24 mL). The post-procedure bloating was complained by 40% patients of group S and 56% patients of group C [Table/Fig-6].
Comparison of amount of flush, duration and post-procedure bloating of upper gastrointestinal endoscopy. p-value # calculated with Pearson Chi-Square test, *calculated with unpaired student’s t-test.
| Group | Time taken for completion (min) Mean±SD | Amount of flush used (mL) Mean±SD | Post-procedure bloating |
|---|
| Present (N) | Absent (N) |
|---|
| Group S | 7.95±0.88 | 8.57±23.04 | 615 | 925 |
| Group C | 8.17±0.98 | 12.24±26.84 | 768 | 609 |
| p-value | 0.0005* | 0.0005* | 0.0005# |
Discussion
This case-control prospective study is the first study of a preparatory solution containing simethicone for gastroscopy to be performed in Indian population. There was significant improvement in mucosal visibility of group S compared to group C. Hence, the findings of this study are similar to other studies that demonstrated improvements in gastric mucosal visibility with a pregastroscopy drink containing a defoaming agent. Secondary outcome measures demonstrated a marked reduction in the time taken for completion of the procedure; Post-procedural bloating and volume of flush required achieving adequate mucosal views in the group S receiving the active pregastroscopy drink. The present study showed that liquid simethicone solution was more effective in reducing obscuring foam and bubbles at all areas of upper gastrointestinal tract, enhancing endoscopic visibility.
According to literature, the effectiveness of simethicone in pre-procedure preparation for EGD was evaluated in few studies, and most studies evaluated the effectiveness of simethicone in preparation regimens of colonoscopy or capsule endoscopy [6,11,12]. A study by Keeratichananont S et al., included 121 patients for EGD and received simethicone (2 mL)+ 60 mL of water or placebo solution + 60 mL of water. They concluded that the mucosal visibility was clear in the simethicone group, but could not affect the duration of procedure [13]. This efficacy in increasing the mucosal visibility was also reported in a meta-analysis study [6].
Post-procedural bloating was statistically higher in group C patients compared to group S. Mean time taken for completion of the procedure was significant statistically in group C. The reduction in time taken for completion of procedure was seen in other study using simethicone by Ahsan M et al., [14]. Mean amount of flush used was 8.57 mL in simethicone group where as it was 12.24 mL in control group with a p-value of 0.0005 which was highly significant statistically. This decrease in amount of flush was also reported by Chang CC et al., and Neale JR et al., but N-acetylcysteine was used in combination along with simethicone [15,16]. The mean TMVS was 5.38 in group S where as it was higher with a value of 6.85 in group C with a p-value of 0.0001 which was highly significant statistically.
The use of this preparatory solution is now standard practice in Japan and the Far East before gastroscopy [17]. They have yet to be widely utilised in the west and India. Cost of this pre-procedure drink is Rs. 6 per patient, which is representing very little extra cost in the context of benefits from this with marked improvement in mucosal visibility with less amount of flush required and faster completion time with less Post-procedural bloating.
Limitation(s)
This was not a double blinded randomised control trial which would have been ideal. The present study design might have bias in mucosal visibility scoring as it is highly subjective. Four observers were involved in this study though all were trained in endoscopy procedure.
Conclusion(s)
This study is first of its kind in India demonstrated that pre-procedure preparation with 125 mg simethicone + 60 mL of water, 30 minutes before the procedure was associated with improved overall endoscopic mucosal visibility. Its also associated with improved mucosal visibility at each of the 4 locations, reduced the need for endoscopic flushing, faster completion time and less Post-procedural bloating. In future, a double blinded randomised control trial can be planned in India to confirm the findings of this study and make this pre-procedure anti-foaming drink a standard for all EGDs in India to improve the mucosal visibility.
GOV 1: Gastricoesophageal Varices extending along the lesser curvature of the stomach; GOV 2: Gastroesophageal Varices extending along the greater curvature towards the gastric fundus; PHG: Portal hypertensive gastropathy; MW: Mallory weiss