With increasing medical interventions, immunosuppressants, hospital stay and antibiotic use; people are finding increased cases of hospital acquired infections. Bacteremia and sepsis are important cause of morbidity and mortality in tertiary health care centres. Majority of bacteremia are caused by the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp (ESKAPE) pathogens. However, in some cases hospital environment bacteria can cause bacteremia and sepsis, especially in immunocompromised patients. Each bacterium is unique with specific antibiotic susceptibilities. So, accurate identification up to species level is important for management of infections. Automated culture and identification systems like VITEK and Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) helps a lot in these processes. Here, the authors presents a case series of bacteremia caused by rare bacteria; namely Campylobacter coli, Sphingomonas paucimobilis, Paenibacillus thiaminolyticus and Cuprividus gilardii. All bacteria were grown in bactec culture and identifications were confirmed by MALDI-TOF MS and drug susceptibility testing was done by VITEK-2. All the patients were having co-morbidities and antibiotic history. Patients responded to guided antibiotic therapy. The authors reiterate that increased suspicion of infection by these bacteria, especially in bacteremia cases of patients have co-morbidities (like agammaglobulinemia, end stage renal disease, diabetes mellitus, kidney failure, etc). Latest identification techniques like VITEK and MALDI-TOF MS should be utilised for diagnosis and treatment of these infections.
Introduction
Bacteremia in humans is not only caused by the ESKAPE pathogens but also by some rare opportunistic environmental pathogens. This case series highlights these rare opportunistic environmental pathogens causing blood stream infections.
Campylobacter jejuni and Campylobacter coli are one of the major causes of gastroenteritis in developed and developing countries. Rare cases of bacteremia have been reported by these species in contrast to Salmonella spp. which are frequently isolated from systemic invasive illnesses [1-3]. Bloodstream infections of Campylobacter spp. are usually seen in immunocompromised patients [4,5]. Studies show that humoral immunodeficiency carries a much higher risk of extraintestinal manifestations by these species [4,5]. Sphingomonas paucimobilis is usually found in nature, soil and drinking water. Nosocomial infections are usually acquired from indwelling catheters or by contaminated fluids [6-8]. This rare pathogen has been implicated to cause bacteremia in immunocompromised hosts [8-10].
Paenibacillus spp is an aerobic, spore forming, gram positive bacteria normally present in the environment like soil and water. This pathogen has been implicated in food poisoning, systemic infections and some cases of bacteremia [11-16]. Cupriavidus gilardii (C. gilardii) is an aerobic, gram-negative, glucose-non fermenting rod sporadically isolated from blood, cerebrospinal fluid, bone marrow and respiratory tract [17,18]. Various cases of C. gilardii have been identified in aplastic anaemia and leukaemia [18-20].
Case 1: Case Report of Campylobacter coli in X-Linked Agammaglobulinemia Patient
A five-year-old boy with normal birth and developmental history presented with fever for last three months. The patient had recurrent history of fever and nasal discharge since two years of age. He also had recurrent history of loose stools since three years of age which was treated with antibiotics like norfloxacin, gentamycin and metronidazole. There was some associated discharge from left ear which got improved with cefixime and amoxicillin-clavulanic acid. The patient had a strong family history of early death of maternal uncles and one male sibling. X-linked Agammaglobulinemia was confirmed in the patient by his previous admission history and he was admitted for evaluation of fever.
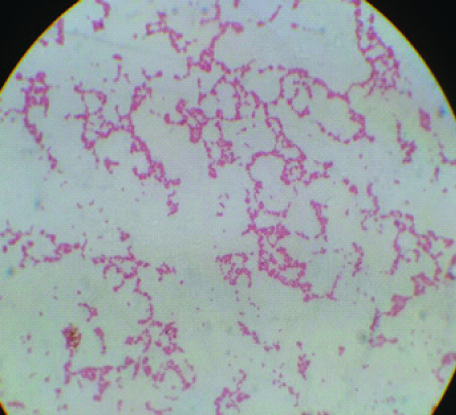
The haematological as well as other biochemical tests were done for further evaluation [Table/Fig-1]. His Bactec blood culture bottle flagged positive after 24 hours of receiving in the laboratory and direct Gram stain revealed multiple curved and spiral gram-negative bacilli [Table/Fig-2]. It was then subcultured on Blood agar and MacConkey agar plates. Non-lactose fermenting colonies were isolated on MacConkey media. Biochemical tests were performed and the organism was oxidase positive, catalase positive, nitrate not reduced, glucose not utilised, hippurate negative and indoxyl acetate-positive. Finally, MALDI-TOF MS was done for confirmation and the final identification was Campylobacter coli. The patient’s stool culture however did not grew Campylobacter coli. Real Time Polymerase Chain Reaction (Fast Track Diagnostics, Luxembourg) on the same stool specimen was positive for Campylobacter coli. The patient was subsequently treated with antibiotics according to the sensitivity pattern tested by VITEK-2 system and reported as per CLSI guidelines [21]. IV ampicillin 1-2 gm every 4-6 hours and IV gentamicin 3 mg/kg/day every eight hours for eight days was started. Oral kanamycin 750 mg four times a day was also initiated to combat the fecal carriage. The patient responded to the antibiotics and his clinical condition improved after five days. Subsequent, blood cultures taken on 6th and 8th days were sterile. Patient was transferred to the Immunology department on the 12th day for further evaluation and work-up for X-linked agammaglobulinemia. Routine injections of IV Immunoglobulins were started at dose of 400 mg/kg given every 3-4 weeks. The patient was discharged in stable state at 17th day with routine follow-ups to be done in Outpatient Department (OPD).
| Case/Patient | Age | Risk factors/Comorbidities/Diagnosis (on previous case reports) | Organism isolated (MALDI-TOF) | Haematological findings | Clinical/Biochemical findings | Antibiogram results | Treatment | Outcome |
|---|
| 1 | 5 years | X-linked Agammaglobulinemia | Campylobacter coli | Hb= 8.7 g/ dL;WBC count= 4.3 × 103 cells/mL with 70% neutrophils, 12% lymphocytes. | Hyperthermia (39.2° C), Tachycardia (122/minute), hypotension (80/52 mmHg),Axillary lymphadenopathy.Liver and kidney function tests within normal limits. | Sensitive to ampicillin, gentamicin, ceftazidime, amikacin, cefoperazone-sulbactam, levofloxacin, imipenem, meropenem. | IV ampicillin 1-2 gm every 4-6 h and IV gentamicin 3 mg/kg/day every 8 h for 8 days was started with Oral kanamycin 750 mg 4 times a day | The patient was discharged in stable state with routine injections of IV Ig very month. |
| 2 | 22 years | Pontine glioma with 38 weeks of pregnancy | Sphingomonas paucimobilis | Hb= 10.2 g/ dL; TLC = 14.9 × 103 cells/mL | Grossly deranged liver and kidney function tests | Sensitive to ceftazidime, amikacin, cefoperazone-sulbactam, levofloxacin, imipenem, meropenem, piperacillin-tazobactam. | Intravenous imipenem 250 mg every 6 hourly plus levofloxacin IV 500 mg once daily for 14 days. | The patient was stable and was discharged as LAMA as could not be operated for Glioma. |
| 3 | 38 years | Kidney failure | Paenibacillus thiaminolyticus | Hb= 9.2 g/ dL; TLC = 13.4 × 103 cells/mL | Grossly deranged liver and kidney function tests | Sensitive to cefixime, ceftazidime, polymyxin, vancomycin, amikacin, cefoperazone-sulbactam, levofloxacin, imipenem, trimethoprim-sulfamethoxazole | Intravenous imipenem 4 g/day and cefixime 200 mg BD for 10 days. | The patient was discharged post Kidney transplant in stable condition |
| 4 | 56 years | End stage renal disease with diabetes mellitus | Cupriavidus gilardii | Hb =8.3 g/dl;TLC=15.8 × 103 cells/mL | Liver and kidney function tests within normal limits. | Sensitive to cefepime, ofloxacin, piperacillin/tazobactam, levofloxacin, ciprofloxacin, aztreonam, imipenem, piperacillin, ceftazidime. | Piperacillin/tazobactam was added to the antibiotic regime as 3.375 g IV q6hr for 4-7 d | Patient was discharged after Brachiocephalic AV fistula procedure in a stable state with dietary advices and antibiotics for one week. |
Gram stain of colonies of campylobacter coli showing curved, Gram negative bacilli.
Case 2: Case Report of Sphingomonas paucimobilis in a Pregnant Female with Pontine Glioma
A 22-year-old female patient presented to the Maternal and Reproductive Health (MRH) OPD of hospital at 38 weeks of pregnancy with history of weakness of bilateral lower limbs since last two months. The patient was diagnosed to have pontine glioma after neurosurgery consultation. Emergency caesarean section was planned and a low birth weight baby of 2.36 kg was delivered. Postoperatively, patient was shifted to ICU.
Patient started deteriorating on postoperative day 4 and was intubated the same day. Blood samples were sent for routine investigations. Two sets of blood culture bottles were sent for culture too. Routine haematological and biochemical investigations of patient were also done [Table/Fig-1]. Bactec culture bottle flagged positive after 18 hours and direct Gram stain revealed gram-negative bacilli. It was then subcultured on Blood and MacConkey agar plates. MALDI-TOF MS was performed and the final identification was done as Sphingomonas paucimobilis.
Disc diffusion testing of the organism was done according to the CLSI M100 guidelines and confirmed by VITEK-2. It showed susceptibility to ceftazidime, amikacin, cefoperazone-sulbactam, levofloxacin, imipenem and meropenem, piperacillin-tazobactam and resistance to aztreonam [21]. Based on the Antibiotic Sensitivity Testing (AST) pattern, the patient was treated with intravenous imipenem 250 mg every six hourly plus levofloxacin IV 500 mg once daily for 14 days. She responded well to the treatment and was transferred on the 18th day to Neurosurgery department for further management. Preanaesthetic checkup was done and planned for surgical removal of glioma along with radiotherapy. The patient’s relatives insisted for Leave Against Medical Advice (LAMA) due to some financial constraints the very next day and was subsequently discharged on 20th day.
Case 3: Case Report of Paenibacillus thiaminolyticus in a Kidney Transplant Recipient
A 38-year-old male patient was admitted in kidney transplant unit for renal transplantation. Patient was asymptomatic and afebrile. Desensitisation protocol was followed with a combination of IV Immunoglobulin (2 doses of 2 g/kg on day 0 and 30) and Rituximab (1 g on day 15). Prior to surgery immunosuppression was started with Etanercept and Tacrolimus with Basiliximab. Kidney transplant was done the following day. Patient was shifted to postoperative ward on endotracheal tube with mechanical ventilation. The patient became haemodynamically unstable the next day with deteriorating ventilator parameters.
Blood samples were sent for routine investigations [Table/Fig-1]. Two sets of blood culture bottles were taken for culture too. Bactec culture bottle flagged positive after 22 hours and direct gram stain revealed gram positive, spore forming bacilli. It was then subcultured on Blood and MacConkey agar plates. MALDI-TOF MS was performed and the final identification was done as Paenibacillus thiaminolyticus.
Disc diffusion testing of the organism was done according to the CLSI M100 guidelines and confirmed by VITEK-2 [21]. It showed susceptibility to cefixime, ceftazidime, polymyxin, vancomycin, amikacin, cefoperazone-sulbactam, levofloxacin, imipenem, trimethoprim-sulfamethoxazole. The patient was treated with intravenous imipenem 4 g/day and cefixime 200 mg BD for 10 days. His general condition started improving and he was extubated after two days with stable haemodynamics and ventilatory parameters. Daily dressing was done and the patient was discharged in stable condition on postoperative 17th day.
Case 4: Case Report of Cupriavidus gilardii in an End Stage Renal Disease Patient
A 56-year-old male was referred from a peripheral hospital to the urology department with diagnosis of end stage renal disease. The patient had history of Diabetes mellitus since 20 years and hypertension since 18 years. He was on Anti-Tubercular Treatment (ATT) for six months and also suffered one episode of seizure two months back.
Colour Doppler revealed thrombosis of left upper limb cephalic vein and examination showed distension of left cephalic vein at elbow with mild tenderness. The patient underwent left Brachiocephalic arterio venouus fistula under local anesthesia and he was shifted to postoperative ward. On postoperative day 2; the haemodynamic parameters of the patient started deteriorating. His routine blood investigations were done [Table/Fig-1]. Blood Bactec culture bottles were sent for culture in pairs. They flagged positive after 16 hours. Direct gram stain revealed gram negative bacilli. The bottles were subcultured on Blood and MacConkey agar plates. MALDI-TOF MS was performed and the final identification was done as Cupriavidus gilardii. Vitek- 2 was used for AST. The isolate was resistant to meropenem, amikacin, rifampin and ampicillin, while susceptible to cefepime, ofloxacin, piperacillin/tazobactam, levofloxacin, ciprofloxacin, aztreonam, imipenem, piperacillin and ceftazidime. The clinician was informed about the susceptibility profile and piperacillin/tazobactam was added to the antibiotic regime as 3.375 g IV q6hr for 4-7 days. The patient responded well to the therapy and improved haemodynamically. Patient was discharged on 12th day in a stable state with dietary advices and antibiotics for one week.
Discussion
There are many bacteria in hospital environment which rarely cause infections. However, they cause bacteremia and other infections in immunocompromised patients. Each bacterium is unique with specific antibiotic susceptibilities. So, accurate identification up to species level is important for management of infections. Especially, bacteremia and sepsis patients require rapid diagnosis and management of infectious causes. Automated systems like VITEK and MALDI-TOF MS play a crucial role in the identification and sensitivity testing of these rare pathogens.
C. coli and C. jejuni are the most frequently isolated Campylobacter species causing diarrhea. Bacteremia caused by these species is usually rare and mostly seen in inmmunocompromised hosts. In patients with Agammaglobulinemia, Campylobacter spp. bacteremia was associated with an impaired antibody response. Several case reports of X-linked agammaglobulinemia with Campylobacter spp. bacteremia have been studied [22]. Stool cultures may or may not grow the causative agent. In this case; stool culture was itself negative but Real time Polymerase chain reaction (PCR) was positive for Campylobacter spp. Intestinal carriage of the organism may be responsible for recurrent bacteremia in these immunocompromised patients.
Bacteraemia with Campylobacter spp. is rare and the frequency is <1% of enteric Campylobacter spp. infections. Patients with invasive campylobacteriosis are with extremes of age and have higher co-morbidity in comparison with enteritis patients with a concomitant negative blood culture. Outcome in these patients with Campylobacteriosis is generally favourable. Suitable antimicrobial therapy with an aminoglycoside usually suffices in these cases.
S. paucimobilis is an opportunistic pathogen and causes infection in immunodeficient patients. It possesses a distinct sphingoglycolipid in the cell wall and does not have the usual lipopolysaccharide component [23]. People with comorbidities such as diabetes mellitus, malignancy, alcoholism, liver cirrhosis, end-stage renal disease, chronic obstructive pulmonary disease and AIDS are much more prone to S.paucimobilis infection [23]. Cases of infection in immunocompetent patient have also been seen [24,25]. S.paucimobilis has been known to be a rare causative agent of bacteremia, catheter-related sepsis, meningitis, peritonitis, osteomyelitis, endophthalmitis and visceral abscesses [24,25]. The patient in this case was immunocompetent but had grossly deranged liver function tests. There are no definitive guidelines for antibiotic therapy for S. paucimobilis infections; hence treatment is individualised according to the in vitro susceptibility profile of clinical isolate in the patient. Studies recommend the use of imipenem or aminoglycoside with a third-generation cephalosporin as the initial treatment of choice in S. paucimobilis infections [23-25]. In the present case also, the patient responded well with imipenem and levofloxacin.
Paenibacillus spp. is mainly environmental in origin, but increasing incidences of bacteremia have led to their isolation from blood. Isolation from cerebrospinal fluid, pleural fluid and urine have also been been noted [26,27]. Several species have been implicated for bacteremia like P.thiaminolyticus, P.konsidensis, P.alve, P.polymyxa, P.larvae. In this case, the patient showed bacteremia due to Paenibacillus spp. which could be due to the environmental contamination of clips and clamps. These findings could further be strengthened by these two cases of bacteremia from identified medical devices as the source (i.e., a hip prosthesis and a heart valve) [26,27].
To the best of knowledge, the present case represents the first clinical infection due to C.gilardii in a patient with end stage renal disease. C.gilardii causes infections in immunocompetent as well as immunocompromised patients. In the previous case reports of infection by C. gilardii, the patients were relatively young (7, 12 and 36-year-old) [28,29]. However, in this case the patient was a middle-aged man of 56 years and underwent left Brachiocephalic AV fistula. Although, the patient had no obvious immunodeficiency, other co-morbidities might have affected the expression of pathogenicity of C. gilardii. In addition, it was possible that creation of AV fistula could have promoted the colonisation of the organism and the development of infection. C. gilardii has intrinsic resistance to many antimicrobial agents. The isolates of C.gilardii were often found to be resistant to aztreonam, meropenem, gentamicin and tobramycin and susceptible to trimethoprim-sulfamethoxazole and tetracycline; as this was evident with previous as well as present case scenario [28,29]. The organism was reported to have the ability to acquire resistance to antibiotics upon their continuous administration [29].
In this case, the strain was susceptible to cefepime, ofloxacin, piperacillin/tazobactam, levofloxacin, ciprofloxacin, aztreonam, imipenem, piperacillin and ceftazidime. The patient responded well to the therapy of piperacillin-tazobactam and improved haemodynamically. Hence, it is of utmost importance to change the antibiotics of the patient according to the recent antimicrobial sensitivity pattern.
Conclusion(s)
Bacteremia with Campylobacter species, Sphingomonas paucimobilis, Paenibacillus thiaminolyticus and Cupriavidus gilardii are rare and an under-reported events.
This is the first case report of Paenibacillus thiaminolyticus from a renal transplant recipient and also Sphingomonas paucimobilis in immunocompetent patient. Increasing incidences of isolation of these organisms in both the immunocompetent as well as immunocompromised individuals are of concern. It requires high index of suspicion on the part of microbiologists for accurate diagnosis and reporting in high risk patients. Finally, this study stresses upon the point of accurate, timely diagnosis as well as treatment of rare under-reported pathogens causing bacteremia.
Ethical considerations: Informed consent was obtained from all the patients and their legal guardians (in case of minors) regarding the publication of images and clinical information in the journal. They were informed of the confidentiality of the data, however, the anonymity cannot be guaranteed.
[1]. Allos BM, Campylobacter jejuni infections: Update on emerging issues and trendsClin Infect Dis 2001 32:1201-06.10.1086/31976011283810 [Google Scholar] [CrossRef] [PubMed]
[2]. Butzler JP, Campylobacter, from obscurity to celebrityClin Microbiol Infect 2004 10:868-76.10.1111/j.1469-0691.2004.00983.x15373879 [Google Scholar] [CrossRef] [PubMed]
[3]. Schønheyder HC, Søgaard P, Frederiksen W, A survey of Campylobacter bacteremia in three Danish counties, 1989-1994Scand J Infect Dis 1995 27:145-48.10.3109/003655495090189957660078 [Google Scholar] [CrossRef] [PubMed]
[4]. Pigrau C, Bartolome R, Almirante B, Planes AM, Gavalda J, Pahissa A, Bacteremia due to Campylobacter species: Clinical findings and antimicrobial susceptibility patternsClin Infect Dis 1997 25:1414-20.10.1086/5161279431389 [Google Scholar] [CrossRef] [PubMed]
[5]. Akiba T, Akiba K, Suto N, Kumagai K, Sakamoto M, Yazaki N, Campylobacter coli bacteremia in an 11-year-old boyPediatr Int 2002 44:543-44.10.1046/j.1442-200X.2002.01595.x12225560 [Google Scholar] [CrossRef] [PubMed]
[6]. Dervisoglu E, Meric M, Kalender B, Sengul E, Sphingomonas paucimobilis peritonitis: A case report and literature reviewPerit Dial Int 2008 28:547-50.10.1177/08968608080280052318708553 [Google Scholar] [CrossRef] [PubMed]
[7]. Holmes B, Owen RJ, Evans A, Malnick H, Willcox WR, Pseudomonas paucimobilis, a new species isolated from human clinical specimens, the hospital environment and other sourcesInt J Syst Bacteriol 1977 27:133-46.10.1099/00207713-27-2-133 [Google Scholar] [CrossRef]
[8]. M-ozdemir M, Pekcan S, Demircili ME, Taşbent FE, Feyzioğlu B, A rare cause of bacteremia in a pediatric patient with Down syndrome: Sphingomonas paucimobilisInt J Med Sci 2011 8:537-39.10.7150/ijms.8.53721960744 [Google Scholar] [CrossRef] [PubMed]
[9]. Toh HS, Tay HT, Kuar WK, Weng TC, Tang HJ, Risk factors associated with Sphingomonas paucimobilis infectionJ Microbiol Immunol Infect 2011 44:289-95.10.1016/j.jmii.2010.08.00721524965 [Google Scholar] [CrossRef] [PubMed]
[10]. Ryan MP, Adley CC, Sphingomonas paucimobilis: A persistent Gram-negative nosocomial infectious organismJ Hosp Infect 2010 75:153-57.10.1016/j.jhin.2010.03.00720434794 [Google Scholar] [CrossRef] [PubMed]
[11]. Noskin GA, Suriano T, Collins S, Sesler S, Peterson LR, Paenibacillus macerans pseudobacteremia resulting from contaminated blood culture bottles in a neonatal intensive care unitAm J Infect Control 2001 29:126-29.10.1067/mic.2001.11153511287883 [Google Scholar] [CrossRef] [PubMed]
[12]. Teng JL, Woo PC, Leung KW, Lau SK, Wong MK, Yuen KY, Pseudobacteraemia in a patient with neutropenic fever caused by a novel paenibacillus species: Paenibacillus hongkongensis sp. novMol Pathol 2003 56:29-35.10.1136/mp.56.1.2912560460 [Google Scholar] [CrossRef] [PubMed]
[13]. Ko KS, Kim YS, Lee MY, Shin SY, Jung DS, Peck KR, Paenibacillus konsidensis sp. nov., isolated from a patientInt J Syst Evol Microbiol 2008 58:2164-68.10.1099/ijs.0.65534-018768623 [Google Scholar] [CrossRef] [PubMed]
[14]. Roux V, Fenner L, Raoult D, Paenibacillus provencensis sp. nov., isolated from human cerebrospinal fluid, and Paenibacillus urinalis sp. nov., isolated from human urineInt J Syst Evol Microbiol 2008 58:682-87.10.1099/ijs.0.65228-018319478 [Google Scholar] [CrossRef] [PubMed]
[15]. Rieg S, Martin Bauer T, Peyerl-Hoffmann G, Held J, Ritter W, Wagner D, Paenibacillus larvae bacteremia in injection drug usersEmerg Infect Dis 2010 16:487-89.10.3201/eid1603.09145720202425 [Google Scholar] [CrossRef] [PubMed]
[16]. McSpadden Gardener BB, Ecology of Bacillus and Paenibacillus spp. in agricultural systemsPhytopathology 2004 94:1252-58.10.1094/PHYTO.2004.94.11.125218944463 [Google Scholar] [CrossRef] [PubMed]
[17]. Coenye T, Falsen E, Vancanneyt M, Hoste B, Govan JR, Kersters K, Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. novInt J Syst Bacteriol 1999 49(Pt 2):405-13.10.1099/00207713-49-2-40510319461 [Google Scholar] [CrossRef] [PubMed]
[18]. Vandamme P, Coenye T, Taxonomy of the genus Cupriavidus: A tale of lost and foundInt J Syst Evol Microbiol 2004 54(Pt6):2285-89.10.1099/ijs.0.63247-015545472 [Google Scholar] [CrossRef] [PubMed]
[19]. Coenye T, Vandamme P, LiPuma JJ, Infection by Ralstonia species in cystic fibrosis patients: Identification of R. pickettii and R. mannitolilytica by polymerase chain reactionEmerg Infect Dis 2002 8(7):692-96.10.3201/eid0807.01047212095436 [Google Scholar] [CrossRef] [PubMed]
[20]. Wauters G, Claeys G, Verschraegen G, De Baere T, Vandecruys E, Van Simaey L, Case of catheter sepsis with Ralstonia gilardii in a child with acute lymphoblastic leukemiaJ Clin Microbiol 2001 39(12):4583-84.10.1128/JCM.39.12.4583-4584.200111724891 [Google Scholar] [CrossRef] [PubMed]
[21]. CLSI. Performance standards for Antimicrobial Susceptibility testing. M 100: 30th ed; January 2020 [Google Scholar]
[22]. Tokuda K, Nishi J, Miyanohara H, Sarantuya J, Iwashita M, Kamenosono A, Relapsing cellulitis associated with Campylobacter coli bacteremia in an agammaglobulinemic patientPediatr Infect Dis J 2004 23:577-79.10.1097/01.inf.0000130080.86862.d515194845 [Google Scholar] [CrossRef] [PubMed]
[23]. Maragakis LL, Chaiwarith R, Srinivasan A, Torriani FJ, Avdic E, Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl1Emerg Infect Dis 2009 15:12-18.10.3201/eid1501.08105419116043 [Google Scholar] [CrossRef] [PubMed]
[24]. Lin JN, Lai CH, Chen YH, Sphingomonas paucimobilis bacteremia in humans: 16 case reports and a literature reviewJournal of Microbiology, Immunology and Infection 2010 43(1):35-42.10.1016/S1684-1182(10)60005-9 [Google Scholar] [CrossRef]
[25]. Pascale R, Russo E, Esposito I, Leone S, Esposito S, Sphingomonas paucimobilis osteomyelitis in an immunocompetent patient: A rare case report and literature reviewNew Microbiologica 2013 36(4):423-26. [Google Scholar]
[26]. Roux V, Raoult D, Paenibacillus massiliensis sp. nov., Paenibacillus sanguinis sp. nov. and Paenibacillus timonensis sp. nov., isolated from blood culturesInt J Syst Evol Microbiol 2004 54:1049-54.10.1099/ijs.0.02954-015280268 [Google Scholar] [CrossRef] [PubMed]
[27]. Ouyang J, Pei Z, Lutwick L, Dalal S, Yang L, Cassai N, Case report: Paenibacillus thiaminolyticus: A new cause of human infection, inducing bacteremia in a patient on hemodialysisAnn Clin Lab Sci 2008 38:393-400. [Google Scholar]
[28]. Tena D, Losa C, Medina MJ, Sáez-Nieto JA, Muscular abscess caused by Cupriavidus gilardii in a renal transplant recipientDiagn Microbiol Infect Dis 2014 79(1):108-10.10.1016/j.diagmicrobio.2014.01.02324582579 [Google Scholar] [CrossRef] [PubMed]
[29]. Karafin M, Romagnoli M, Fink DL, Howard T, Rau R, Milstone AM, Fatal infection caused by Cupriavidus gilardii in a child with aplastic anemiaJ Clin Microbiol 2010 48(3):1005-07.10.1128/JCM.01482-0920071544 [Google Scholar] [CrossRef] [PubMed]