The emergence of Multidrug Resistant (MDR) organism is a major global issue, associated with several diseases such as bloodstream infections, pneumonia, skin and soft tissue infections, surgical site infections, urinary tract infections, bone and joint infections, ear and ocular infections, meningitis and so on [1]. Multidrug Resistant Gram Negative Bacteria (MDR-GNB) are one of the important cause of increased mortality and morbidity [2]. Colistin have been re-established as last resort antibiotics for the management of this MDR gram negative pathogen [3]. However, resistance to colistin has been emerged in recent past [4]. This requires the urgent need for standardised susceptibility testing method for colistin, both for healthcare and surveillance purposes. According to joint The Clinical Laboratory Standards Institute (CLSI) - EUCAST guidelines, the only valid method to test the Minimal Inhibitory Concentration (MIC) of colistin is the BMD [5,6]. But, this gold standard reference method is too laborious and time consuming to perform in a clinical laboratory [7-9]. BMD is rarely done in microbiology laboratories as routine basis for clinical isolates [10]. Though disc diffusion and gradient diffusion are the widely used methods for AST at clinical laboratories [9,11], they remain as challenging method for colistin as this antibiotic poorly diffuses into the agar medium and it was not approved for colistin by both CLSI and EUCAST [5,6,12-14]. Due to this property of poor diffusibility, E-test gradient diffusion showed lower MIC among the resistant isolates leading to false sensitive reports [15]. Taking into account of the increasing usage of colistin, a reliable and easy to perform as well as rapid method is needed to assess the colistin susceptibility. To reduce the difficulties encountered with reference BMD methods, many commercial BMD kits such as SensiTest™, UMICTM, MICRONAUTTM are available in the market in which procedure time is much shorter than the reference method and also produces reliable results [10,15-17]. This study has evaluated the performance of Erba Mannheim MIKROLATEST colistin MIC testing kit which is one of the commercially available testing kits and compared it with the reference inhouse BMD method. But, this commercial kit require the same incubation time (16-18 hours) as that of the reference method to get the results. Rapid polymyxin NP test developed by Nordmann P et al., detects colistin resistance in the presence of fixed concentration of polymyxins in less than two hours [18]. This method is based on the principle of bacterial growth which is identified by colour change of the culture medium due to metabolisation of glucose by bacteria [18]. As this new rapid method is easy to perform, evaluation of this method with the standard reference method is essential.
The present study have evaluated the degree of agreement and different types of errors of colistin susceptibility testing methods such as commercial BMD testing kit and rapid polymyxin NP test with the reference in-house BMD method.
Materials and Methods
A comparative analytical study was done in Department of Microbiology in Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India from July 2018 to July 2019. During this period, the routine samples, such as Blood, Endotracheal aspirate, Sterile fluids (bile, peritoneal fluid, CSF), tissue bit and pus sample which were requested for culture and sensitivity reports by the clinicians were subcultured in blood agar and MacConkey agar and the organisms growing in these media were subjected to AST. The isolates of Escherichia coli, Klebsiella pneumoniae, Enterobacter species and Citrobacter species showing resistance to Carbapenem were collected for the study. Organisms from Enterobacteriaceae family showing intrinsic resistance to colistin such as Proteus, Serratia, Morganella and Providencia were excluded. Repeated isolates from the same patient were excluded from the study.
The study was carried out on bacterial isolates that were isolated from clinical samples which are routinely isolated in the hospital laboratory and informed consent was not taken from the patients as a part of institute’s policy. The study did not involve any human subjects directly and the study was approved by JIPMER institute Ethics Committee (JIP/IEC/2018/0123).
Sample size of carbapenem resistant Enterobacteriaceae was calculated to be 294 isolates based on previous year data obtained from department register which contains reports of blood culture isolates, respiratory, sterile fluids, pus and tissue bit isolates. The isolates were collected till the sample size was reached and all the three procedures such as in-house BMD method (the reference method), commercial colistin BMD method obtained from MIKROLATEST ®MIC Erba Mannheim (This Invitrodiagnostic kit obtained CE marking, comply with the Directive 98/79/EC) and Rapid polymyxin NP test were done simultaneously [18,19].
The reference inhouse BMD was performed according to joint CLSI-EUCAST recommended guidelines and ISO 20776:2006 [19,20]. The cation adjusted Mueller-Hinton broth (Sigma-Aldrich 90922) was prepared following manufacturer’s instructions. The stock solution of colistin was prepared from colistin sulphate salt (sigma -aldrich C4461-100MG). The final bacterial inoculum size of 5×105 CFU/mL was used. The test was done in triplicate in polystyrene Microtitre plate (Corning CLS3585 flat bottom 96 wells with lid) and incubated for 16 to 20 hours at 35°C and examined visually by two observers and MIC values were noted. For quality control, ATCC 25922 Escherichia coli and NCTC Escherichia coli 13846 (mcr-1 positive) were used as recommended by EUCAST [21]. For sterility control, physiological saline was added to wells instead of bacterial inoculum. Because the CLSI does not provide clinical breakpoints for colistin for Enterobacteriaceae [6], European Committee on Antimicrobial Susceptibility Testing (EUCAST) MIC breakpoints was used for interpretation: <2 susceptible and >2 resistant [Table/Fig-1] [5].
Colistin interpretative breakpoints according to the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines in 2018 [5,6].
| Family | CLSI clinical breakpoints (μg/Ml) | CLSI Epidemiological cut off value (μg/Ml) | EUCAST breakpoints (μg/Ml) | CLSI recommended quality control |
|---|
| S | I | R | Wildtype | Nonwildtype | S | I | R | |
|---|
| Enterobacteriaceae | Not available | ≤2 | >2 | <2 | | >2 | ATCC 25922 (E.coli) MIC 0.25 -2 μg/mL |
S- Susceptible; I- intermediate; R- Resistant
The test method using the commercial kit, Erba Mannheim MIKROLATEST colistin MIC testing method was done in parallel. The procedure was done according to manufacturer’s instructions and the inoculated strips were incubated for 16 to 20 hours at 35°C. MIC was examined visually by two independent observers and results were interpreted as mentioned before. ATCC 25922 Escherichia coli and NCTC Escherichia coli 13846 (mcr-1 positive) were used as quality control strains [21]. This kit will detect colistin MIC in the range 0.25 μg/mL to 16 μg/mL. If the MIC value was <0.25 μg/mL, the value 0.25 μg/mL was retained. Similarly, if the MIC values were above 8 μg/mL, results were interpreted as 16 μg/mL for the ease of result interpretation [16].
Rapid Polymyxin NP Test
Rapid polymyxin NP test was performed based on protocol provided by Nordmann P et al., [18]. The stock solution of colistin sulphate (Sigma Aldrich) was prepared to obtain colistin concentration of 0.2 mg/mL. The rapid polymyxin NP solution was prepared which consists of 2.5% MHB-CA powder, 1% anhydrous glucose and 0.005% phenol red indicator. Then Rapid polymyxin NP solution was prepared separately without colistin and with colistin (the final colistin concentration in the rapid NP solution- 5 μg/mL). The test was performed in a 96-well microtitre plate.
The bacterial inoculum of 3.0-3.5 McFarland standard optical density (~109 CFU/mL) was prepared for both test and control strains and the final colistin concentration in the wells was 3.75 μg/mL. The plate was incubated at 35±2°C and inspected for 2 hours at the end of 4 hours. The test isolate was considered as colistin resistant if the isolates have grown in the presence of colistin which is evident by change in the colour of the medium from reddish orange to yellow in colour. The isolate is considered colistin sensitive if medium remained the initial orange colour [Table/Fig-2].
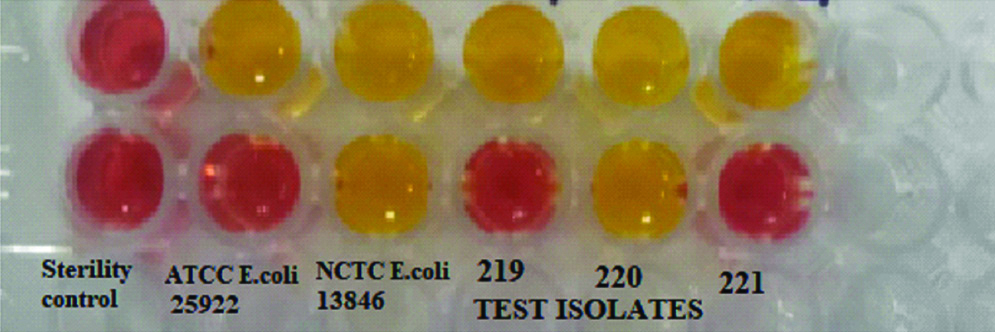
Interpretation: If susceptibility result of the isolate done by the test method is same as the reference standard method (if test method result is resistant, reference method is also resistant), the test method is said to be categorically agreed with the reference method [22], otherwise it is categorically disagreed. Categorical disagreement is classified into Very Major, Major and Minor errors. If the test method belongs to sensitive category and the reference method is resistant, it is very major error. If the test method belongs to resistant category and the reference method is sensitive, it is major error [22]. If the test method is intermediate category and the reference method is either Sensitive or resistant category, it is said to be Minor error. As EUCAST didn’t give any intermediate breakpoint for colistin, Minor errors is not applicable for this antibiotic [5]. Essential agreement is defined as MIC plus or minus one fold dilution of the reference MIC [22]. As the Rapid polymyxin NP test shows whether the isolate is Sensitive or Resistant to colistin and MIC results could not be obtained with this method, essential agreement is not applicable for this method [18].
Statistical Analysis
The statistical analysis was carried out by kappa statistics using Stata version 14 software. Categorical and Essential agreement between commercial BMD method and reference BMD method was interpreted according to ISO 20776-2:2007 guidelines [22]. Kappa value between commercial BMD method and gold standard inhouse BMD method was found to be 0.78 with p-value <0.001. Categorical agreement was calculated for Rapid polymyxin NP test with the reference in-house BMD method [22]. Kappa value between Rapid polymyxin NP test and gold standard inhouse BMD method was found to be 0.72 with p-value <0.001. Their corresponding Sensitivity, Specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) were analysed.
Results
Among the 294 consequetively collected Carbapenem resistant Enterobacteriaceae isolates, blood culture isolates accounts for 35% (n=103), isolates of endotracheal aspirate 25% (n=74); sterile fluids isolates (bile, pleuralfluid, peritoneal fluid) 10% (n=29); isolates from tissue bit samples contributes to 18% (n=53) and pus sample isolates is 12% (n=35). Overall, among 294 clinical isolates Klebsiella pneumoniae was the most common isolate (66%) [Table/Fig-3].
Distribution of carbapenem resistant enterobacteriaceae isolates.
| Organism | Number of isolates tested (%) |
|---|
| Klebsiella pneumoniae | 195 (66.3) |
| Escherichia coli | 67 (22.7) |
| Enterobacter spp. | 23 (7.8) |
| Citrobacter spp. | 9 (3) |
Susceptibility results of 294 isolates which were evaluated for three methods showed the MIC range for quality control strains within acceptable limits, 0.5-2 μg/ml for ATCC Escherichia coli 25922 and 4 μg/ml-8μg/ml for NCTC Escherichia coli 13846 on all occasions. Colistin MIC distribution for 294 isolates with reference BMD method shown in [Table/Fig-4].
Colistin MIC distribution with reference BMD method for 294 isolates.
| Organism | No. of isolates tested | MIC range (μg/mL) |
|---|
| 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
|---|
| Klebsiella pneumoniae | 195 | 5 | 9 | 34 | 54 | 43 | 14 | 11 | 16 | 6 | 3 |
| Escherichia coli | 67 | 1 | 4 | 21 | 19 | 18 | 1 | 1 | 0 | 1 | 1 |
| Enterobacter spp. | 23 | 0 | 2 | 10 | 7 | 2 | 1 | 0 | 0 | 1 | 0 |
| Citrobacter spp. | 9 | 0 | 0 | 0 | 3 | 2 | 1 | 0 | 2 | 0 | 1 |
According to the interpretation, overall for 294 isolates categorical agreement of commercial BMD with reference in-house method was 91.1%. The overall categorical disagreement was found in 8.8%, majority of which were major error (ME, 6.1%) followed by very major error (ME, 2.7%). Agreement and disagreement between commercial testing method with the reference method for individual organism shown in the [Table/Fig-5].
Agreement of commercial brothmicrodilution method with the reference method for individual organism.
| Organism | Categrical agreement n (%) | Categorical disagreement | Essential agreement |
|---|
| Major error n (%) | Very major error n (%) | Total % | Agreed n (%) | Disagreed n (%) |
|---|
| K.pneumoniae (N=195) | 178 (91.2) | 10 (5.1) | 7 (3.5) | 8.6 | 166 (85.12) | 29 (14.87) |
| E. coli (N=67) | 59 (88) | 8 (11.9) | 0 | 11.9 | 53 (79.1) | 14 (20.8) |
| Enterobacter spp. (N=23) | 22 (95.6) | 0 | 1 (4.3) | 4.3 | 18 (78.2) | 5 (21.7) |
| Citrobacter spp. (N=9) | 9 (100) | 0 | 0 | 0 | 8 (88.8) | 1 (11.1) |
n: Number of isolates
The isolates with MIC values (done by reference inhouse BMD method) below 0.25 and above 16 were not included for essential agreement calculation to avoid confusion in comparison and this would not have affected the categorical agreement analysis. The overall essential agreement of commercial BMD kit with reference method was 83.3% and 16.6%. The overall sensitivity and specificity of this commercial kit to detect colistin resistance were 86.2% and 91.9% respectively. PPV and NPV were 72% and 96% respectively.
With the Rapid colistin NP test, categorical agreement was 92.5% and the categorical disagreement was 7.4% which includes major error of 6.1% and very major error of 1.3%. Sensitivity, specificity, PPV and NPV of this method to detect colistin resistance were 93.1%, 92.3%,75% and 98% respectively. Agreement and disagreement between Rapid polymyxin NP test with the reference method for individual organism shown in the [Table/Fig-6].
Agreement of rapid polymyxin np test with the reference method for individual organism.
| Organism | Categorical agreement | Categorical disagreement |
|---|
| Major error, n (%) | Very major error, n (%) | Total % |
|---|
| K.pneumoniae (N=195) | 181 (92.8) | 11 (5.6) | 3 (1.5) | 7.1 |
| E.coli (N=67) | 61 (91) | 5 (7.4) | 1 (1.4) | 8.80 |
| Enterobacter spp. (N=23) | 22 (95.6) | 1 (4.3) | 0 | 4.30 |
| Citrobacter spp. (N=9) | 8 (88.8) | 0 | 1 (11.1) | 11.10 |
n: Number of isolates
Discussion
The use of colistin has been increased in recent times to treat patients with gram negative MDR pathogens. But, susceptibility testing methods of colistin is challenging due to many reasons such as poor diffusion in agar, cationic property of colistin and heteroresistance in MDR organism [9]. As CLSI and EUCAST has not recommended disc diffusion, there are no currently established zone diameter breakpoints [5,6]. MIC clinical breakpoints are provided by CLSI for Pseudomonas and Acinetobacter spp. CLSI provides epidemiological cut-off which is the MIC breakpoints below (<2 μg/mL) where strains are described as wild type and strains with MIC above are described as non-wild type strain (>2 μg/mL). In most of the studies, EUCAST clinical breakpoints were followed for interpretation MIC of <2 μg/mL is considered susceptible and >2 μg/mL resistant [14,18].
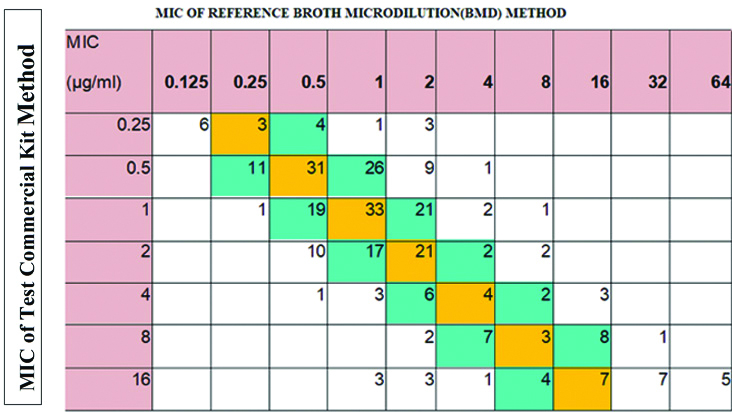
Compared to the reference inhouse BMD method, the commercially available BMD kits are easy to use and they are less expensive also. In this study, Erba Mannheim colistin MIC kit was used, to be validated, this commercial kit must met the following criteria: Categorical Agreement (CA) ≥90%, Essential Agreement (EA) ≥90%, Major Error (ME) ≤3% and Very Major Error (VME) ≤3%. The test kit has satisfied categorical agreement which was 91.1% which consists of very major error 2.7%. As both CLSI and EUCAST does not provide intermediate category for colistin, the errors may be very major or major error, [8] high major error of 6.1% was obtained. But, the essential agreement with reference method was not fulfilled. MIC Correlation of Commercial kit method with the reference method is shown in the [Table/Fig-7].
Correlation of commercial bmd kit(erba mannheim-mikrolatest) with reference inhouse BMD method.
Shaded in orange=number of isolates with identical MIC
Shaded in blue =MICs within essential agreement (within one fold dilution)
Studies have been done using several commercial colistin BMD kits. In a study by Matuschek E et al., [10], categorical agreement for different bacterial isolates done by different BMD products varied from 89 to 95% which is similar to the present study. Some BMD products such as SensiTest™ and MICRONAUT™ products showed high essential agreement 96-99%, but some BMD kits like SensiTest™ and UMIC™ showed essential agreement of 88% and 82% respectively, which is close to the present study results [8]. In a study by Bardet L et al., categorical agreement was 100% and essential agreement of 94% compared with the reference BMD method [16]. In a study done by Jayol A et al., [17] comparison of three BMD products obtained categorical agreement of 97.8% for SensiTest™, 91.1% for UMIC kit and Microscan panel and essential agreement of these BMD panels with the reference method were not mentioned.
The original article of Rapid polymyxin NP test done by Nordmann P et al., showed the sensitivity and specificity of this new method, 99.3% and 95.4% respectively with the reference method [18]. Jayol A et al., studied 123 enterobacterial isolates and found the sensitivity and specificity of 98.8% and 97.5% respectively [23]. Poirel L et al., had performed Rapid polymyxin NP test in several isolates from Enterobacteriaceae family and obtained sensitivity and specificity of 100% [24]. In another study by Yainoy S et al., done in 339 Enterobacteriaceae isolates, sensitivity was 100% and specificity was 95.9% [Table/Fig-8] [25]. The results of the rapid polymyxin test done on 294 Enterobacteriaceae isolates showed the sensitivity of 93.1% and the specificity of 92.3% and the categorical disagreement accounts for 6.1% and 1.3%, major and very major error respectively. These results exhibited good performance of this Rapid polymyxin NP test with short turnaround time similar to the previous results. This could be useful for resource poor settings as the materials involved with this test can be obtained from routine day to day laboratory consumables. A study done by Simar S et al., on 143 Enterobacter isolates showed sensitivity of 25%, which was attributed to the presence of heteroresistance among those isolates [26]. As the present study included only 23 Enterobacter isolates, the phenomenon of hetoresistance and skipped wells could have been missed. Therefore, further studies are needed before implementation of this method.
Results of rapid polymyxin np test obtained in various studies.
| Various study | Total isolates tested | Test sensitivity (%) | Test specificity (%) |
|---|
| Nordmann P et al., [18] 2016 | 200 | 99.3 | 95.4 |
| Jayol A et al., [23] 2018 | 123 | 98.8 | 97.5 |
| Poirel L et al., [24] 2018 | 105 | 100 | 100 |
| Yainoy S et al., [25] 2018 | 339 | 100 | 95.9 |
| Present study | 294 | 93.1 | 92.3 |
Limitation(s)
The main limitation of this study is that it is purely laboratory based, as study involved only bacterial isolates. The study didn’t clinically correlate the test results with the patient outcome as the patient details were not analysed in this study. Molecular studies could be done in these clinical isolates to determine the mechanism of underlying colistin resistance which is another limitation of the study.
Conclusion(s)
The Rapid Polymyxin NP test can be used as rapid screening method to determine susceptibility or resistance to colistin which can be confirmed by one of the reliable, less laborious and reproducible commercial BMD kit such as Erba Mannheim MIKROLATEST kits, after proper validation of the test products in clinical laboratories. This in turn will be useful for the physicians in prior administration of the drug, thereby mortality and morbidity can be decreased in healthcare centres.
Declaration: The paper was presented by SD at Microcon 2019 conference held in Mumbai from November 29, 2019 to December 1, 2019.
S- Susceptible; I- intermediate; R- Resistant
n: Number of isolates
n: Number of isolates