Introduction
The adhesion of bacteria to implanted devices in the human body is a major risk factor that can affect the long-term success of these devices. Staphylococcus epidermidis (S. epidermidis) have few virulence factors which have been identified and reported including autolysin (AtlE), Polysaccharide Intercellular Adhesin (PIA), Fibrinogen Binding Protein (SdrG) and Lipase enzymes (GehC and GehD). These virulence factors play an important role in the pathogenesis of S. epidermidis.
Aim
To investigate the role of some of the virulence factors of S. epidermidis in the adhesion to three different types of intravascular cannulae used in hospitals.
Materials and Methods
Three different types of most commonly used intravascular cannulae in hospitals were tested in this study. Polytetrafluoroethylene (PTFE), Siliconized Polytetrafluoroethylene (SPTFE) and Vialon cannulae were incubated with different S. epidermidis strains, including the mutant strains that were deficient in a specific virulence factor, each separately. The virulence factors tested with each strain were AltE, PIA, SdrG and Lipase enzymes (GehC and GehD). The number of detached bacteria were determined by plating serial dilutions on agar plates. Experiments were repeated and expressed as means and standard deviations with at least three experiments. Results were computed and analysed using Statistical Package for Social Sciences (SPSS) version-20 program. Tabulated values were compared with a student’s t-test. Statistical significance was defined as p<0.05.
Results
All three types of intravascular cannulae showed significant reduction in adhesion by means of cfu/mL with the mutant strains deficient in adhesion factors when compared to the wild strains. The reduction in the percentages of adhesion of AtlE-strain when compared to wild type strain were 35% for PTFE, 38% for SPTFE and 48% for Vialon cannula. This was statistically significant. PTFE (p-value=0.005), SPTFE (p-value=0.02), and Vialon (p-value=0.001). Likewise, all three types of cannulae showed reduction that was statistically significant except for one mutant strain which was deficient in GehC lipase. However, GehD lipase showed statistically significant reduction in adhesion of bacterial colonies similar to mutant strains for PIA and SdrG indicating a role of these virulence factors in the process of adhesion to biomaterials.
Conclusion
The virulence factors of S. epidermidis; AtlE, PIA, SdrG and Lipase GehD, play an important role in the adhesion to intravascular cannulae. Further researches are needed to investigate the involvement of other virulence factors in order to understand and prevent such mechanism.
Introduction
Adhesion process of bacteria to implanted devices in the human body is a major risk factor that can affect the long-term success of these devices. These devices can increase life expectancy and give better health outcomes to patients [1]. However, there is a risk of device related infection, which is an infection related to any implanted device in the human body such as catheters and cannulae. It is however difficult to avoid such infections as local bacterial infection in presence of implanted devices can occur even with minimum number of bacteria when compared to the situation where there is no implanted device in the body. The minimum bacterial dose to cause pus by intradermal injection has already been demonstrated in human volunteers [2]. Although of all available studies on bacterial colonisation and adhesion to medical devices, mechanism of the process of adhesion and colonisation is not fully understood [3-8]. For infection to occur, the bacteria have to come very close to the surface of the device. This usually happens with the help of many factors including Vander Waals, hydrophobic and electrical forces. Once the bacteria is on top of the surface there will be a biochemical interaction with the surface and bacteria. The ability of bacteria to produce specific components such as virulence factors may help in bacterial adhesion and colonisation of the surface of the device [9,10]. Intravenous cannulae are routinely used during hospital admission for various ailments and to facilitate treatment. Previously, it has been shown that two bacteria known to cause skin infections; Staphylococcus epidermidis (S. epidermidis) and Staphylococcus aureus (S. aureus) can adhere to Polytetrafluoroethylene (PTFE), Siliconized Polytetrafluoroethylene (SPTFE) and Vialon cannulae materials in a process which is time dependent [11].
The formation of biofilm by bacteria on the surfaces of implanted device like the intravenous cannulae in the human body complicates the situation and makes the treatment of device related bacterial infection difficult. Biofilms protect the bacteria from external environmental factors, immune system and antibiotics [12]. The metabolic activity of bacteria inside biofilms also changes rendering the situation more difficult. The main virulence activity of S. epidermidis comes from its ability to produce Exopolymeric Substances (EPS) to help it in adhesion to biomaterials and forming biofilms [13]. S. epidermidis is known as the major pathogen involved in medical device infections. More than 22% of the collected infective samples in Intensive Care Units (ICU) in United States are related to S. epidermidis [14]. This bacterium is also considered as one of the most frequent blood culture contaminant [15]. Catheters which are used in hospitalised patients are considered the most common cause of bacteremia in patients and the causative organism is mainly coagulase-negative staphylococci such as S. epidermidis. This bacterium is responsible for more than 50% of these catheter related infections [16].
Biofilm formation by S. epidermidis is considered as one of the important virulence factors by which the bacteria can evade the host defense mechanism and cause infections. Different stages have been described in the process of formation of biofilm starting with the primary attachment of bacteria to the formation of multi-layered cell clusters with cell-cell adhesion. S. epidermidis can attach directly to the surface of the implant material or to matrix proteins which coat the device surface and originate from the host [17].
It was demonstrated recently that S. epidermidis biofilms have a huge number of cells that are called persistent cells which protect the bacteria from neutrophils and immune system [18]. PIA which is controlled by the ica operon is another virulence factor of S. epidermidis that has been shown to play an important role as a virulence factor for this bacterium. Another virulence factor which is important in accumulation of S. epidermidis biofilm is the biofilm associated protein Bap/Bhp which is considered a surface adhesion protein [19]. S. epidermidis also have the Poly-γ-Glutamic Acid (γ-PGA) which may have a role in evading host defense by affecting the phagocytosis process [20]. It has been shown that S. epidermidis has enterotoxin like toxin which may be part of the virulence mechanism of this bacterium. Delta toxin is also produced by S. epidermidis and can cause cytolytic activity to lyse blood cells and induce bleeding [21]. Phenol Soluble Modulins (PSMs) which are able to lyse white and red blood cells; and further induce cytokines production are found in S. epidermidis. The most important PSMs in this bacteria was found to be the cytolysin which is one of the six element PSMs identified in S. epidermidis [22]. An extracellular matrix protein called Embp was also identified in S. epidermidis. This protein is an important factor as a fibronectin binding protein which helps S. epidermidis in forming biofilm as well as its adhesion to biomaterials [23].
Another virulence factor of S. epidermidis is the production of lantibiotic which may play an important role in the colonisation of this bacterium on skin and mucous membrane. Many other proteins are produced by S. epidermidis including cell wall associated and cytoplasmic associated proteins such as the 42 kDa protein which is a cell wall glyceraldehydes-3-phosphate dehydrogenase and the 32 kDa cytoplasmic membrane lipoprotein. These proteins may have a role in S. epidermidis pathogenicity [24]. It has been shown that S. epidermidis can produce many enzymes that help the bacteria to colonise and evade host defense mechanism. Examples of these enzymes are lipases such as the GehC and GehD lipase which are thought to be part of the virulence mechanism of S. epidermidis [25-27]. All these virulence factors are controlled by the genetics of these bacteria. In S. aureus, most of the extracellular and surface associated virulence factors are controlled by the accessory gene regulator (agr). In the past few years, S. epidermidis has become a very important organism in medical device related infection. Intervascular cannulae (catheters) are used routinely in hospital for drug and fluid delivery for admitted patients. S. epidermidis is an important skin commensal, so all such catheters are subjected to be colonised by such organism after their insertion. The purpose of this study was to examine the ability of S. epidermidis to adhere to different types of cannulae made of different materials. At the same time this study aimed to investigate the role of different S. epidermidis virulence factors in the role of adhesion of this organism to intravascular cannulae.
Materials and Methods
The present in-vitro study was conducted at the College of Dentistry Research Center Laboratories, King Saud University; Riyadh, Saudi Arabia during October 2019 and January 2020. The manuscript was a part of the PhD thesis for which an ethical and an institutional approval was granted.
Bacterial Strains and Growth
S. epidermidis strains that were used in this study include S. epidermidis strains O-47 and its mutant strain deficient in AtlE and mutant strain deficient in PIA. The other strain used was S. epidermidis strain HB and its mutant strain deficient in fibrinogen binding protein (SdrG). Finally, S. epidermidis strain 9 and its mutant strains which are deficient in production of lipase enzymes were used. These include S. epidermidis strain 2J24 deficient in GehC lipase enzyme (GehC mutant), and strain KIC82 deficient in GehD lipase enzyme (GehD mutant). All the strains were obtained from Department of Microbiology, King Saud University.
S. epidermidis strain 9 which was used in the current study is a wild type strain isolated from volar forearm [26]. Its isogenic mutants deficient in GehC (2J24) or GehD (KIC82) are otherwise identical to the wild-type strain, with minimal introduction of foreign genetic material and were modified using allele replacement mutagenesis [28]. Strain O-47 is a biofilm-positive clinical isolate and its AtlE isogenic mutant Strain is a Tn917 insertional mutant that is deficient in AtlE production. The other O-47 mutant strain is a Tn917 insertional mutant that is deficient in PIA production because of interruption of the ica locus [29]. Strain HB is an isolate from a patient with osteomyelitis [30]. This strain is positive in SdrG which is a 119-kDa surface protein on S. epidermidis which mediates adhesion to fibrinogen. Its isogenic mutant is deficient in this important protein [31].
Cannulae Materials
Three different types of the most commonly used intravascular cannulae in hospitals were tested in this study. The first, PTFE, second the SPTFE and third; Vialon cannula. The PTFE material is a fluorocarbon solid which is cosidered as a high-molecular-weight material. The SPTFE material has a high resistance to changes and also possesses a good tissue compatibility. The Vialon material is a special biomaterial which was developed in the past years especially for human intravascular access for fluid and drug delivery. It applies minimum pressure on the blood vessel walls and this effect avoids the risk of vessel penetration. The three cannulae were cut into two centimeters length and then heated at the end of each cannula to close its end to avoid bacteria from entering inside the lumen. Cannulae were then sterilised using ethylene oxide (EtO) method [32,33].
Adhesion Assay
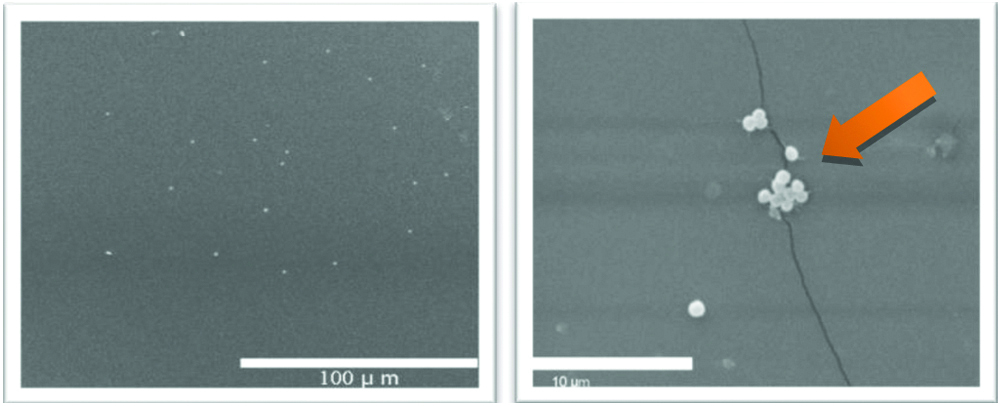
The two-centimeter sterile intravascular cannula was incubated with different S. epidermidis strains including the mutant strains that are deficient in specific virulence factor, each separately. The piece of cannula was incubated with each bacterium in four milliliters of phosphate buffered saline. The number of bacteria added were determined based on the dilution curve for each strain by measuring the abosrbance at wave length 650 nm using the spectrophotometer. A total of 108 cell/mL bacteria were used in adhesion experiment for each strain. After two hours incubation period of bacterial strains and different cannulae at a temperature of 37°C, non-adhered bacteria which were present in the medium were removed by washing the cannula three times with phosphate buffered saline. The liquid from the washing steps of cannulae was investigated for bacteria by plating on agar plates. In order to remove the adherent bacteria on intravascular cannulae, the cannulae samples were resuspended again into four mL of phosphate buffer saline with Tween 20 and vortexed to detach any attached bacteria to the surface of the cannuale. Vortexing was done for three minutes. The number of detached bacteria were determined by plating serial dilutions on agar plates [11,33]. To take an average of the results these experiments were repeated at least three times. Numbers of bacteria were calculated as Colony Forming Units (CFU)/mL. To calculate bacterial adhesion in percentage, CFU/mL of bacteria adhered to each type of cannulae were divided by the CFU/mL of wild strain (control) adhered to the same cannula and multiplied by 100. Then the difference in adhesion percentages between different strains was calculated by subtracting from 100. [Table/Fig-1a,b] show the adhesion of S. epidermidis (strain O47) to PTFE intravascular cannula under scanning electron microscope image.
Scanning electron microscope (SEM) images of adhesion of S.epidermidis (wild strain O47) to the PTFE intravascular cannula at lower and higher magnification. The arrow shows the area of adhesion in the form of bacterial colonies.
Statistical Analysis
Data were computed and analysed using the SPSS v20 with a student’s t-test. Significance was defined as <0.05. Data were expressed as means and standard deviations of at least three experiments.
Results
S. epidermidis Autolysin (AtlE) plays a role in bacterial adhesion to cannulae materials
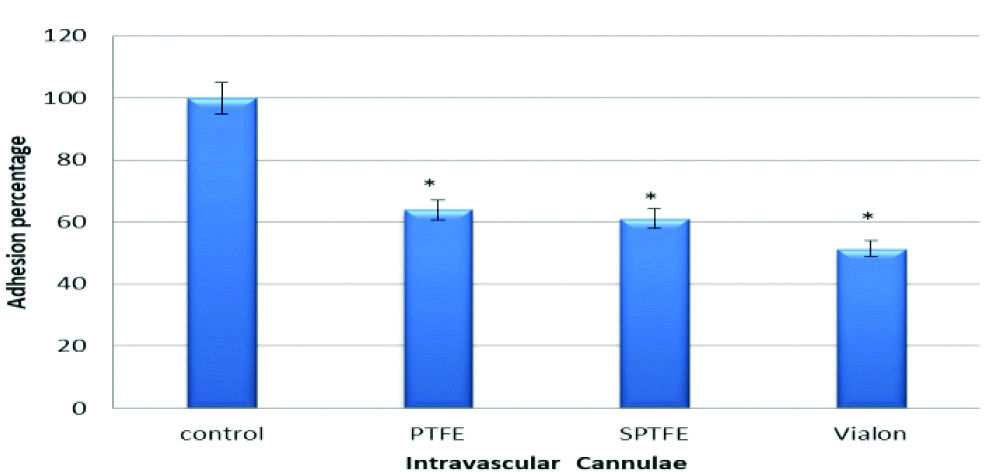
To investigate the role of autolysin (AtlE) in adhesion of S. epidermidis to PTFE, SPTFE and Vialon intravascular cannulae, two S. epidermidis strains were used. The first one; strain O-47 and the second its isogenic mutant which is deficient in autolysin (O-47 AtlE). The results in this experiment showed that there was a significant reduction in adhesion of S. epidermidis to PTFE (p-value=0.005), SPTFE (p-value=0.02), and Vialon (p-value=0.001), intravascular cannulae. The reduction percentages of adhesion of O-47 AtlE negative strain when compared to the O-47 wild type strain were 35% for PTFE, 38% for SPTFE and 48% for Vialon cannula respectively. This reduction in adhesion indicates the role of AtlE in adhesion process of S. epidermidis to these cannulae. The results are represented by [Table/Fig-2].
Adhesion of S. epidermidis strain O-47 isogenic mutant deficient in Autolysin (AtlE) to three types of Intravascular cannulae. Data are the percentages of adhesion to different cannulae compared to the control (100% adhesion of wild type strain O47). The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. The graph shows that S. epidermidis mutant strain deficient in autolysin have less adhesion capacity.
*indicates that p-value is less than 0.05
S. epidermidis Polysaccharide Intercellular Adhesin (PIA) is involved in adhesion to cannulae materials
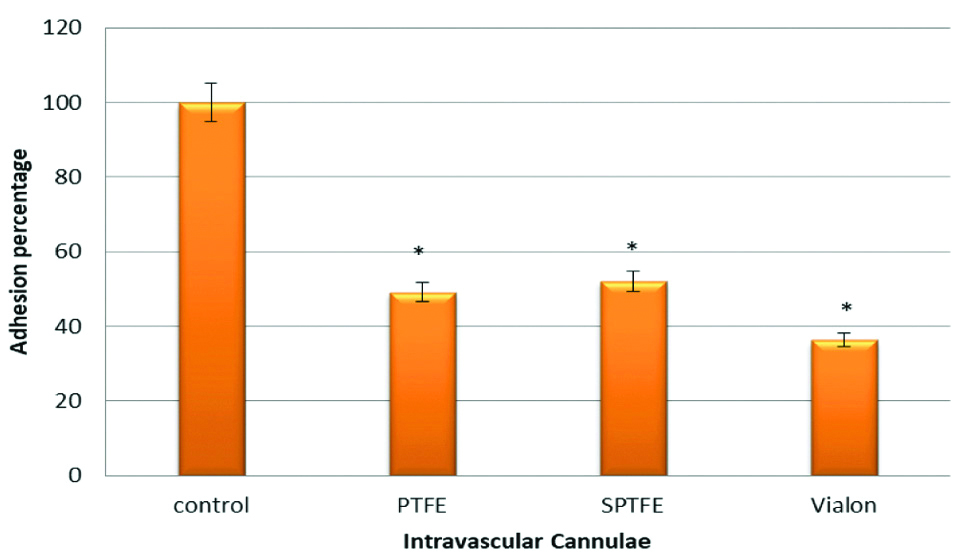
To investigate the role of PIA in adhesion of S. epidermidis to PTFE, SPTFE and Vialon intravascular cannulae, two S. epidermidis strains were used. The first one; strain O-47 and the second its isogenic mutant which is deficient in PIA (O-47 PIA). The results in this experiment showed that there was a significant reduction in adhesion of S. epidermidis to PTFE (p-value=0.001), SPTFE (p-value=0.01), and Vialon (p-value=0.0001), intravascular cannulae. The reduction percentages of adhesion of O-47 PIA negative strain when compared to O-47 wild type strain were 50% for PTFE, 47% for SPTFE and 63% for Vialon cannula, respectively. Disruption of PIA in mutant strain resulted in this reduction in adhesion and this indicates the role of the PIA in adhesion process of S. epidermidis to these cannulae. The results are represented in [Table/Fig-3].
Adhesion of S. epidermidis strains O-47 isogenic mutant deficient in Polysaccharide Intercellular Adhesin (PIA) to three types of Intravascular cannulae. Data are the percentages of adhesion to different cannulae compared to the control (100% adhesion of wild type strain O-47). The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. The graph shows that S. epidermidis mutant strain deficient in PIA have less adhesion capacity.
*indicates that p-value is less than 0.05
S. epidermidis Fibrinogen Binding Protein (SdrG) is involved in adhesion to cannulae materials
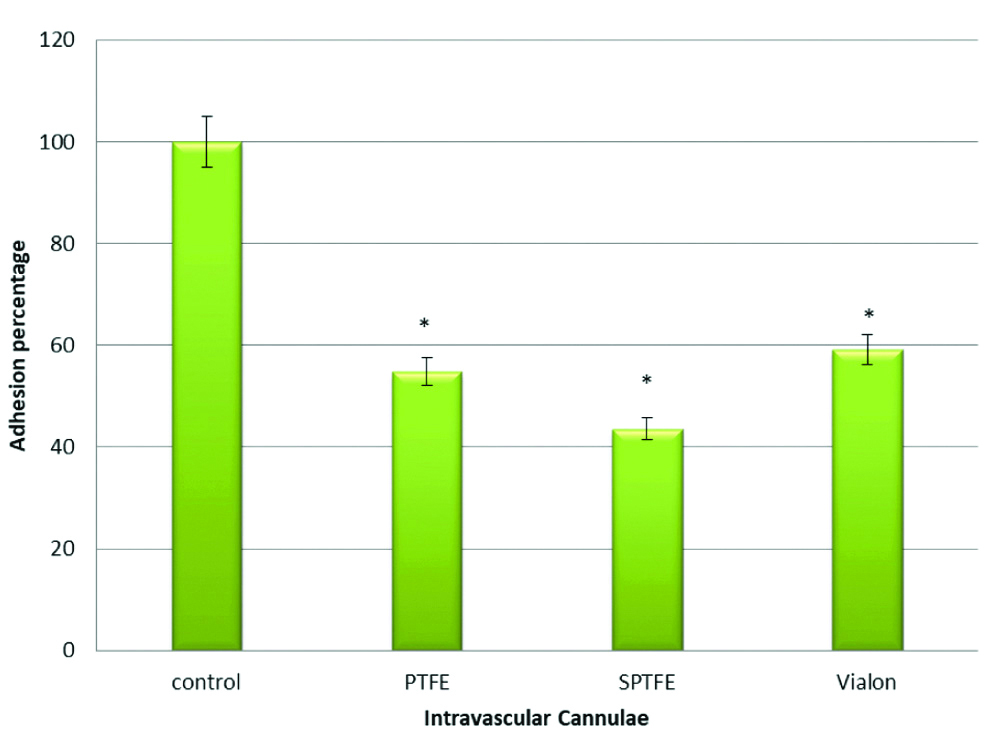
To investigate the role of fibrinogen binding protein (SdrG) in adhesion of S. epidermidis to PTFE, SPTFE and Vialon intravascular cannulae, two S. epidermidis strains were used. The first one; strain HB and the second its isogenic mutant which is deficient in SdrG protein (HB SdrG). The results in this experiment showed that there was a significant reduction in adhesion of S. epidermidis HB SdrG strain to PTFE (p-value=0.003), SPTFE (p-value=0.001), and Vialon (p-value=0.0003), intravascular cannulae. The reduction percentages of adhesion of HB SdrG negative strain when compared to HB wild type strain were 45% for PTFE, 56% for SPTFE and 40% for Vialon cannula, respectively. This reduction in adhesion highlights the role of the SdrG protein in adhesion process of S. epidermidis to these cannulae. The results are represented in [Table/Fig-4].
Adhesion of S. epidermidis strains HB isogenic mutant deficient in Fibrinogen Binding Protein (SdrG) to three types of Intravascular cannulae. Data are the percentages of adhesion to different cannulae compared to the control (100% adhesion of wild type strain HB). The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. The graph shows that S. epidermidis mutant strain deficient in SdrG protein have less adhesion capacity.
*indicates that p-value is less than 0.05
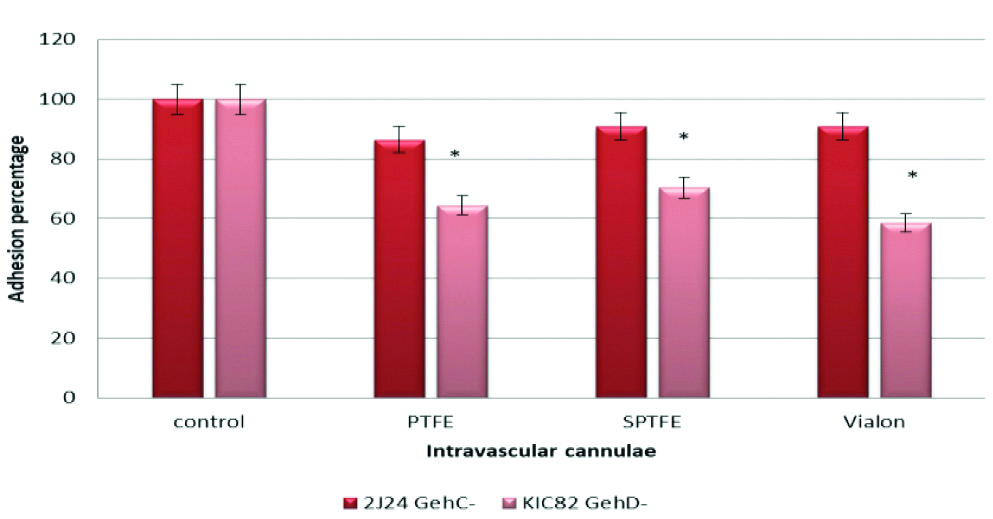
GehD Lipase is involved in adhesion of S. epidermidis to cannulae materials
To investigate the role of lipase enzymes (GehC and GehD) in adhesion of S. epidermidis to PTFE, SPTFE and Vialon intravascular cannulae, three S. epidermidis strains were used. The first one; strain 9 and the second is isogenic mutant which is deficient in GehC lipase (2J24) and the third strain which is deficient in GehD lipase (KIC82). The results in this experiment showed that there was insignificant reduction in adhesion of S. epidermidis strain 2J24 deficient in GehC lipase to PTFE (p-value=0.3), SPTFE (p-value=0.4), and Vialon (p-value=0.6), intravascular cannulae. However, there was a significant reduction of adhesion of strain KIC82 deficient in GehD lipase to PTFE cannulae (p-value=0.03) as well as STPFE (p-value=0.01) and Vialon (p-value=0.02) intervascular cannulae. The reduction percentages of strain KIC82 which is deficient in GehD lipase when compared to strain 9 were 35% for PTFE, 29% for SPTFE and 41% for Vialon cannulae. These results are shown in the [Table/Fig-5].
Adhesion of S. epidermidis strain 2J24 deficient in GehC Lipase and strain KIC82 which is deficient in GehD Lipase to three types of Intravascular cannulae. Data are the percentages of adhesion to different cannulae compared to the control (100% adhesion of wild type strain 9). The results are from one representative experiment of at least three; data are the means and standard deviations of three replicate cultures. The graph shows that S. epidermidis mutant strain deficient in GehD lipase have less adhesion capacity.
*indicate that p-value is less than 0.05
Discussion
In the past years, bacterial adhesion to biomaterials has gained increased attention [1]. These device and biomaterials related infections are caused by cerebrospinal fluid shunts, intraspinal pumps, neurostimulators, cochlear implant, prosthetic heart valves, implantable defibrillator and cannulae [34]. Management of such device related infections is difficult and requires the replacement of the device in most cases. Another important risk factor for these infections is the development of bacterial resistance over time [3,4]. Mutant strains which are deficient in some virulence factors were used in the study. The results of experiments showed that virulence factors of S. epidermidis such as AtlE, PIA, SdrG and GehD lipase could play an important role in adhesion of this organism to different types of intravascular cannulae including PTFE, SPTFE and Vialon. SdrG protein of S. epidermidis is an important virulence factor which is responsible for binding of this bacterium to fibrinogen [31]. It has been shown that SdrG from S.epidermidis can promote its adhesion and could be a factor in biomaterials related infections [7,35,36]. Vanzieleghem T et al., has shown that the ability of S. epidermidis to adhere to coated polydimethylsiloxane fibrinogen coated surfaces was enhanced in the presence of abundant cell surface SdrG [36]. Strain which is disrupted in SdrG showed less ability to adhere to PTFE, SPTFE and Vialon cannulae. It has been shown that increased expression of S. epidermidis SdrG promotes adhesion to surfaces [36]. Khalil H et al., found that major adhesin responsible for binding to sutures was AtlE when adhesion of S. epidermidis to surgical sutures was investigated. They further suggested that inhibitors of S. epidermidis AltE may be useful to prevent bacterial adhesion to sutures [33]. Another important virulence factors of S. epidermidis is PIA which have been shown to affect bacterial colonisation and adhesion along with the lipase enzymes such as the GehC lipase and GehD lipase, and AtlE [28,29,37]. The role and involvement of these factors in adhesion of S. epidermidis to intravascular cannulae PTFE, SPTFE and Vialon has not been tested in the literature. The results of the current experiment showed that disruption in the genes responsible for the function of PIA, AtlE or GehD lipase can reduce the adhesion of S. epidermidis to intravascular cannulae. Disruption of AtlE in S. epidermidis strain O-47 showed significant reduction in the adherence of this bacterium to all types of cannulae. The same effect was reported by Rupp ME et al., when they investigated adhesion of S. epidermidis to central venous catheters in rat models [29]. Similar results have been shown in adhesion of S. epidermidis to surgical sutures [33]. The number of adhered bacteria was significantly reduced for S. epidermidis PIA mutant strain indicating its role in adhesion process. In contrast to present study findings, Higashi JM et al., in 1998 found that PIA was not involved in adhesion of S. epidermidis to polyethylene [38]. Similar results have been shown in adhesion of S. epidermidis to epithelial cells [39] and surgical sutures [33]. Therefore, AtlE and PIA could be important determinants for adhesion of S. epidermidis to intravascular cannulae. In future, molecules or drug therapy that can disrupt the role of AtlE and PIA may be good treatment modalities for device related infections.
Another important virulence determinant which S. epidermidis possess are the lipase enzymes. Two S. epidermidis lipases have been identified including the GehC and GehD lipase [26]. The GehD lipase of S. epidermidis has been reported to be a collagen binding adhesin of this organism [25]. In the current study the role of the two S. epidermidis lipases in adhesion process to PTFE, SPTFE and Vialon was investigated. The results showed that the GehD but not the GehC lipase had an important role in adhesion of S. epidermidis to all tested cannulae. The wild types strain 9 which has both GehC and GehD lipases showed higher adhesion capacity to PTFE, SPTFE and Vialon cannulae when compared to the mutant strains 2J24 and KIC82. Such comparison indicates the role of lipase GehD in adhesion of S. epidermidis to different cannulae. This could be due to the second function of GehD lipase as collagen binding adhesin [40]. These results were consistent with that of the findings from the study of Khalil H et al., [33].
Experiments were mainly done with incubation period of two hours and all CFU’s that are presented in most of the graphs were the bacterial adhesion during this period. But to investigate the role of longer incubation periods on adhesion of different strains of S. epidermidis, adhesion of all strains including mutant strains were tested over 12 and 24 hours. The results showed that in all wild strains such as strain O-47, 9 and HB; adhesion was increased significantly over time. In contrast to these findings, adhesion of mutant strains deficient in AtlE, PIA, SdrG and lipase enzymes did not show significant increase of adhered S. epidermidis over longer periods of time. As these virulence factors could be important in the process of adhesion this could be the reason that adhesion process was interrupted even if the incubation period was longer.
Limitation(s)
The current study is an in-vitro study that needs to be tested in-vivo as host factors can play an important role in adhesion of S. epidermidis to intravascular cannulae. The role of more S. epidermidis virulence factors need to be investigated.
Conclusion(s)
The present study done with S. epidermidis virulence factors showed an important role in the adhesion of this organism to intravascular cannulae and subsequently to device related infections. Bacterial adhesion to biomaterials should be taken in consideration when selecting the best material for patient that has less bacterial colonisation over time in order to decrease the risk of device-related infection. Understanding the mechanism of adhesion of this bacterium and the factors that are involved could lead to a solution to all these infections related to medical devices. Interruption of such mechanism could be a crucial way of prevention of such infection.
Author Declaration:
Financial or Other Competing Interests: As declared above
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 26, 2020
Manual Googling: May 11, 2020
iThenticate Software: May 29, 2020 (15%)
[1]. Pacha-Olivenza MA, Rodriguez-Cano A, Gonzalez-Martin ML, Gallardo-Moreno AM, Kinetic of adhesion of S. epidermidis with different EPS production on Ti6Al4V surfacesBiomed Res Int 2019 2019:143780610.1155/2019/143780631915679 [Google Scholar] [CrossRef] [PubMed]
[2]. Elek SD, Conen PE, The virulence of Staphylococcus pyogenes for man. A study of the problems of wound infectionBr J Exp Pathol 1957 38(6):573-86. [Google Scholar]
[3]. Linnes JC, Mikhova K, Bryers JD, Adhesion of Staphylococcus epidermidis to biomaterials is inhibited by fibronectin and albuminJ Biomed Mater Res A 2012 100(8):1990-97.10.1002/jbm.a.3403622566405 [Google Scholar] [CrossRef] [PubMed]
[4]. Lianhua Y, Yunchao H, Guangqiang Z, Kun Y, Xing L, Fengli G, The effect of iatrogenic Staphylococcus epidermidis intercellar adhesion operon on the formation of bacterial biofilm on polyvinyl chloride surfacesSurg Infect (Larchmt) 2014 15(6):768-73.10.1089/sur.2013.12925402758 [Google Scholar] [CrossRef] [PubMed]
[5]. Eroshenko D, Morozov I, Korobov V, The role of plasma, albumin, and fibronectin in Staphylococcus epidermidis adhesion to polystyrene surfaceCurr Microbiol 2015 70(6):846-53.10.1007/s00284-015-0796-825744155 [Google Scholar] [CrossRef] [PubMed]
[6]. Nuryastuti T, Krom BP, Ica-status of clinical Staphylococcus epidermidis strains affects adhesion and aggregation: A thermodynamic analysisAntonie Van Leeuwenhoek 2017 110(11):1467-74.10.1007/s10482-017-0899-228608317 [Google Scholar] [CrossRef] [PubMed]
[7]. Swetha TK, Pooranachithra M, Subramenium GA, Divya V, Balamurugan K, Pandian SK, Umbelliferone impedes biofilm formation and virulence of methicillin-Resistant Staphylococcus epidermidis via impairment of initial attachment and intercellular adhesionFront Cell Infect Microbiol 2019 9:35710.3389/fcimb.2019.0035731681633 [Google Scholar] [CrossRef] [PubMed]
[8]. Borowski RGV, Barros MP, da Silva DB, Lopes NP, Zimmer KR, Staats CC, Red pepper peptide coatings control Staphylococcus epidermidis adhesion and biofilm formationInt J Pharm 2020 574:11887210.1016/j.ijpharm.2019.11887231812797 [Google Scholar] [CrossRef] [PubMed]
[9]. Bouafia N, Chouchene I, Ben CA, Bouchoucha S, Njah M, Risk of mortality due to device associated infectionTunis Med 2015 93(10):638-45. [Google Scholar]
[10]. Duran LW, Preventing medical device related infectionsMed Device Technol 2000 11(6):14-17. [Google Scholar]
[11]. Abusalim G, Alharbi S, Khalil H, Wainright M, Khiyami M, Adhesion of Staphylococcus epidermidis and Staphylococcus aureus to intravascular cannulaeLife Science Journal 2013 10(4):981-88. [Google Scholar]
[12]. Percival SL, Emanuel C, Cutting KF, Williams DW, Microbiology of the skin and the role of biofilms in infectionInt Wound J 2012 9(1):14-32.10.1111/j.1742-481X.2011.00836.x21973162 [Google Scholar] [CrossRef] [PubMed]
[13]. von EC, Peters G, Heilmann C, Pathogenesis of infections due to coagulase-negative staphylococciLancet Infect Dis 2002 2(11):677-85.10.1016/S1473-3099(02)00438-3 [Google Scholar] [CrossRef]
[14]. Otto M, Staphylococcus epidermidis- The ‘accidental’ pathogenNat Rev Microbiol 2009 7(8):555-67.10.1038/nrmicro218219609257 [Google Scholar] [CrossRef] [PubMed]
[15]. David MD, Elliott T, Coagulase-negative staphylococciBr J Hosp Med (Lond) 2015 76(8):C126-28.10.12968/hmed.2015.76.8.C12626255931 [Google Scholar] [CrossRef] [PubMed]
[16]. Al-Tawil ES, Almuhareb AM, Amin HM, Catheter-related blood stream infection in patients receiving long-term home parenteral nutrition: Tertiary care hospital experience in Saudi ArabiaSaudi J Gastroenterol 2016 22(4):304-08.10.4103/1319-3767.18760427488325 [Google Scholar] [CrossRef] [PubMed]
[17]. Gottenbos B, van der Mei HC, Busscher HJ, Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymersJ Biomed Mater Res 2000 50(2):208-14.10.1002/(SICI)1097-4636(200005)50:2<208::AID-JBM16>3.0.CO;2-D [Google Scholar] [CrossRef]
[18]. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM, Microbial biofilmsAnnu Rev Microbiol 1995 49:711-45.10.1146/annurev.mi.49.100195.0034318561477 [Google Scholar] [CrossRef] [PubMed]
[19]. Tormo MA, Knecht E, Gotz F, Lasa I, Penades JR, Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer?Microbiology 2005 151(Pt 7):2465-75.10.1099/mic.0.27865-016000737 [Google Scholar] [CrossRef] [PubMed]
[20]. Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidisJ Clin Invest 2005 115(3):688-94.10.1172/JCI20052352315696197 [Google Scholar] [CrossRef] [PubMed]
[21]. Vuong C, Otto M, Staphylococcus epidermidis infectionsMicrobes Infect 2002 4(4):481-89.10.1016/S1286-4579(02)01563-0 [Google Scholar] [CrossRef]
[22]. Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Staphylococcus epidermidis strategies to avoid killing by human neutrophilsPLoS Pathog 2010 6(10):e100113310.1371/journal.ppat.100113320949069 [Google Scholar] [CrossRef] [PubMed]
[23]. Williams RJ, Henderson B, Nair SP, Staphylococcus aureus fibronectin binding proteins A and B possess a second fibronectin binding region that may have biological relevance to bone tissuesCalcif Tissue Int 2002 70(5):416-21.10.1007/s00223-001-2073-z12055657 [Google Scholar] [CrossRef] [PubMed]
[24]. Tang H, Wang A, Liang X, Cao T, Salley SO, McAllister JP III, Effect of surface proteins on Staphylococcus epidermidis adhesion and colonisation on siliconeColloids Surf B Biointerfaces 2006 51(1):16-24.10.1016/j.colsurfb.2006.04.01116806854 [Google Scholar] [CrossRef] [PubMed]
[25]. Bowden MG, Visai L, Longshaw CM, Holland KT, Speziale P, Hook M, Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin?J Biol Chem 2002 277(45):43017-23.10.1074/jbc.M20792120012218064 [Google Scholar] [CrossRef] [PubMed]
[26]. Farrell AM, Foster TJ, Holland KT, Molecular analysis and expression of the lipase of Staphylococcus epidermidisJ Gen Microbiol 1993 139(2):267-77.10.1099/00221287-139-2-2678436947 [Google Scholar] [CrossRef] [PubMed]
[27]. Joseph B, Ramteke PW, Kumar PA, Studies on the enhanced production of extracellular lipase by Staphylococcus epidermidisJ Gen Appl Microbiol 2006 52(6):315-20.10.2323/jgam.52.31517325444 [Google Scholar] [CrossRef] [PubMed]
[28]. Longshaw CM, Farrell AM, Wright JD, Holland KT, Identification of a second lipase gene, gehD, in Staphylococcus epidermidis: Comparison of sequence with those of other staphylococcal lipasesMicrobiology 2000 146(Pt 6):1419-27.10.1099/00221287-146-6-141910846220 [Google Scholar] [CrossRef] [PubMed]
[29]. Rupp ME, Fey PD, Heilmann C, Gotz F, Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat modelJ Infect Dis 2001 183(7):1038-42.10.1086/31927911237828 [Google Scholar] [CrossRef] [PubMed]
[30]. Nilsson M, Frykberg L, Flock JI, Pei L, Lindberg M, Guss B, A fibrinogen-binding protein of Staphylococcus epidermidisInfect Immun 1998 66(6):2666-73.10.1128/IAI.66.6.2666-2673.19989596732 [Google Scholar] [CrossRef] [PubMed]
[31]. Hartford O, O’Brien L, Schofield K, Wells J, Foster TJ, The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogenMicrobiology 2001 147(Pt 9):2545-52.10.1099/00221287-147-9-254511535794 [Google Scholar] [CrossRef] [PubMed]
[32]. Dempsey DJ, Thirucote RR, Sterilization of medical devices: A reviewJ Biomater Appl 1988 3(3):454-523.10.1177/0885328288003003032654354 [Google Scholar] [CrossRef] [PubMed]
[33]. Khalil H, Marraiki NA, Abusalim G, Williams RJ, Nair SP, Adhesion of Staphylococcus epidermidis to surgical suturesBiosciences, Biotechnology Research Asia 2016 8(1):01-10.10.13005/bbra/817 [Google Scholar] [CrossRef]
[34]. Vinh DC, Embil JM, Device-related infections: A reviewJ Long Term Eff Med Implants 2005 15(5):467-88.10.1615/JLongTermEffMedImplants.v15.i5.2016218897 [Google Scholar] [CrossRef] [PubMed]
[35]. Herman P, El-Kirat-Chatel S, Beaussart A, Geoghegan JA, Vanzieleghem T, Foster TJ, Forces driving the attachment of Staphylococcus epidermidis to fibrinogen-coated surfacesLangmuir 2013 29(42):13018-22.10.1021/la402917224111821 [Google Scholar] [CrossRef] [PubMed]
[36]. Vanzieleghem T, Herman-Bausier P, Dufrene YF, Mahillon J, Staphylococcus epidermidis affinity for fibrinogen-coated surfaces correlates with the abundance of the SdrG adhesin on the cell surfaceLangmuir 2015 31(16):4713-21.10.1021/acs.langmuir.5b0036025821995 [Google Scholar] [CrossRef] [PubMed]
[37]. Rupp ME, Fey PD, In vivo models to evaluate adhesion and biofilm formation by Staphylococcus epidermidisMethods Enzymol 2001 336:206-15.10.1016/S0076-6879(01)36591-6 [Google Scholar] [CrossRef]
[38]. Higashi JM, Wang IW, Shlaes DM, Anderson JM, Marchant RE, Adhesion of Staphylococcus epidermidis and transposon mutant strains to hydrophobic polyethyleneJ Biomed Mater Res 1998 39(3):341-50.10.1002/(SICI)1097-4636(19980305)39:3<341::AID-JBM1>3.0.CO;2-J [Google Scholar] [CrossRef]
[39]. Costa AR, Henriques M, Oliveira R, Azeredo J, The role of polysaccharide intercellular adhesin (PIA) in Staphylococcus epidermidis adhesion to host tissues and subsequent antibiotic toleranceEur J Clin Microbiol Infect Dis 2009 28(6):623-29.10.1007/s10096-008-0684-219130107 [Google Scholar] [CrossRef] [PubMed]
[40]. Sakinc T, Woznowski M, Ebsen M, Gatermann SG, The surface-associated protein of Staphylococcus saprophyticus is a lipaseInfect Immun 2005 73(10):6419-28.10.1128/IAI.73.10.6419-6428.200516177313 [Google Scholar] [CrossRef] [PubMed]