Introduction
Anti-Retroviral Therapy (ART) initiation in patients leads to increase in CD4 counts and decrease in the Plasma Viral Load (PVL). However, some patients fail to achieve a significant increase in CD4 count despite undetectable PVL. In spite of complete viral suppression, patients have immunological failure; this is referred as “immunological discordance”. This study is a retrospective analysis of PVL in cases of immunological failure in Human Immunodeficiency Virus (HIV) patients and aims to find out the rate of discordance and associated co-morbid conditions.
Aim
To carry out a retrospective analysis of PVL in HIV patients with immunological failure on ART in a tertiary health care centre in Nagpur, Maharashtra, India.
Materials and Methods
This study was carried out at the ART centre in Government Medical College and Hospital, Nagpur, Maharashtra, India. Patients (>16 years of age) of immunological failure (458) who started second-line ART during the period 2012-2017 were included in the study. The data was coded using MS-Excel 2013 and statistical softwares OpenEpi (Version 3.01) and STATA (Version 10.1-2011, Texas, USA) were used for analysis.
Results
The most common co-existing condition seen was tuberculosis. Risk of low (<100) baseline CD4 count was almost three times higher in males. Risk of immunological failure {in those with greater than 100 cell decrease after six months of Highly Active Antiretroviral Therapy (HAART)} was higher in those with low baseline CD4 counts (0-200 cells/mm3) (OR-1.39). The rate of discordance was 17.82%. The number of patients of immunological failure decreased when ART was initiated at higher CD4 counts.
Conclusion
Discordance was seen in patients of immunological failure, thus, PVL assay must be done before second line ART initiation to avoid unnecessary switching of regimen. Early initiation of ART can lead to a better prognosis, thus helping us reach closer to the 90-90-90 target.
Introduction
The total number of People Living with Human Immunodeficiency Virus (PLHIV) in India is estimated to be 2.1 million in 2018 [1]. The introduction of ART into clinical practice has led to dramatic reductions in morbidity and mortality associated with the HIV infection [2].
Over the years, a change has been seen in the guidelines for initiation of ART depending on the CD4 cell counts of the patients and currently, it is initiated irrespective of the CD4 cell counts [3-6]. Initiation of ART should lead to immune recovery and PVL suppression [7]. An adequate CD4 response is defined as an increase of 50-150 cells/mm3/year with an accelerated response in the first three months of the treatment [8]. However, some patients fail to achieve a significant increase in CD4 count despite undetectable/low PVL (<1000 copies/ml) [1]. They are considered to have an immunological failure despite complete viral suppression and this is referred to as “immunological discordance” [9]. In some patients, a different pattern of discordant response is seen in which there is a sustained CD4+ cell count response even with persistent viraemia [10]. Previous clinical studies demonstrated that the prevalence of immunological discordance to ART ranges from 20% to 30% [10-12].
In 2014, the UNAIDS launched the 90-90-90 target which aims that by 2020, 90% of all people living with HIV will know their HIV status, 90% of these will receive sustained ART and 90% of all people receiving ART will have viral suppression [13]. According to UNAIDS 2017 data for India, 79% of people living with HIV knew their status and 56% of people living with HIV were on treatment [14].
In resource limited settings like India, the cost of treatment is very high and routine virological monitoring and genotyping resistance is not done to start the therapy and see the response to therapy [15]. With CD4 counts as the surrogate marker, immunological failure cases have been reported from various parts of the country [15-19]. However, it is important to assess their PVL to evaluate discordant responses in them, since discordant responders have shown relatively higher risk of progression to AIDS and non-AIDS related mortalities [20]. This will also help in reduction of accumulation of drug resistance mutations and improve patient outcomes by preventing unnecessary switching of regimens. Assessment of PVL will also be useful in the quantification of the third 90 goal of the 90-90-90 target [1].
This study of retrospective analysis of PVL in cases of immunological failure in HIV patients was undertaken with the aim to find out the rate of discordance and associated co-morbid conditions in these patients.
Materials and Methods
This retrospective study was carried out at the ART centre in Government Medical College and Hospital, Nagpur, Maharashtra, India with data collected from the ART cards of the patients. The project was approved by the Institutional Ethics Committee (REG. NO. -ECR/43/inst/MH/2013). According to National Aids Control Organization (NACO) guidelines, immunological failure is defined as fall of CD4 count to pre-therapy baseline (or below) or 50% fall from on treatment peak value (if known) or persistent CD4 levels below 100 cells/mm3, and virological failure is defined as PVL >1,000 copies/mL [1,3]. Out of a total of 5091 patients on ART, 458 patients (>16 years of age) of immunological failure who were started on second-line ART between the time period 2012-2017 (time duration of study- 05/2016 to 12/2017) were included in the study. Patients who died, stopped treatment, transferred out or were lost to follow-up were excluded from the study. Basic demographic details (such as age, sex, weight, marital status, educational status, area of residence and mode of transmission), baseline and follow-up CD4 counts at 6, 12 and 24 months, viral load values, baseline ART regimens and associated conditions (if any) were collected from the cards of patients. The estimation of the CD4 counts was done using the flow-cytometer (BD FACsCalibur) at GMC, Nagpur and whereas that of PVL was done by COBAS TaqMan HIV-1Test (Roche) at JJ group of Hospitals, Mumbai, Maharashtra, India. The percentage of patients of immunological failure with undetected or poor viral load (<1000 cells/cmm) was used to calculate the rate of discordance [1].
Statistical Analysis
The data was coded using MS-Excel 2013 and statistical software OpenEpi: Open Source Epidemiologic Statistics for Public Health (Version 3.01) [21], and STATA (Version 10.1-2011, Texas, USA) were used for analysis and p-value <0.05 was considered to be significant.
Results
The basic demographic factors of patients with immunological failure are shown in [Table/Fig-1]. The male patients were 72.27% (331) and 27.73% (127) were female. Most of the patient were (92.79%) <54 years of age. The different first line ART regimens used in the patients is enlisted in [Table/Fig-2]. Zidovudine was used by 47.82% patients. Male to female ratio was found to be 2.6:1. It was observed that 39.57% males and 20.47% females had baseline CD4 counts <100 cells/mm3 [Table/Fig-3]. This was found to be statistically significant (p-value=0.008). Further, risk of low (<100) baseline CD4 count was found almost three times higher in males. (OR=2.81 95% CI 1.68-4.83, p=0.0001). The common co-existing conditions seen were tuberculosis (22.05%), anaemia (15.07%), candidiasis (14.41%), diarrhoea due to Microspora Isospora Cryptosporidium (MIC) complex (12.23%) etc., [Table/Fig-4]. Out of 458 patients of immunological failure, the PVL of 303 patients was available. The PVL was low (<1000 copies/mL) or undetectable in 54 patients. Hence, the rate of immunological discordance was 17.82% [Table/Fig-5]. Risk of immunological failure (in those with >100 cell decrease after six months of HAART) was high in those with lower baseline CD4 counts (0-200 cells/mm3) (OR=1.39) [Table/Fig-6]. The number of patients of immunological failure decreased when ART was initiated at higher CD4 counts [Table/Fig-7]. The year wise data of the patients has been tabulated in [Table/Fig-8].
Basic demographic factors of patients of immunological failure.
| Variable | Frequency (n=458) | Percentage (%) |
|---|
| Gender |
| Male | 331 | 72.27 |
| Female | 127 | 27.73 |
| Age |
| 16-54 years | 425 | 92.79 |
| >54 years | 33 | 7.21 |
| Weight |
| <40 kg | 64 | 13.97 |
| 40-60 kg | 347 | 75.76 |
| >60 kg | 47 | 10.27 |
| Marital status |
| Married | 335 | 73.14 |
| Widowed | 52 | 11.35 |
| Single | 44 | 9.61 |
| Divorce/Separate | 24 | 5.24 |
| Live-in | 3 | 0.66 |
| Educational status |
| Illiterate | 27 | 5.90 |
| Primary and secondary education | 337 | 73.58 |
| College | 94 | 20.52 |
| Area |
| Urban | 288 | 62.88 |
| Rural | 170 | 37.12 |
| Occupation |
| Housewives | 78 | 17.03 |
| Labourers+Others* | 108 | 23.58 |
| Unemployed | 127 | 27.73 |
| Private job | 40 | 8.73 |
| Business | 29 | 6.33 |
| Drivers | 28 | 6.11 |
| Government Job | 21 | 4.59 |
| Farmers+HH** | 27 | 5.90 |
| Mode of transmission |
| Heterosexual | 376 | 82.10 |
| Unknown | 67 | 14.63 |
| Blood transfusion | 3 | 0.66 |
| MSM*** | 3 | 0.66 |
| PPTCT | 5 | 1.09 |
| CSW† | 4 | 0.86 |
*Others: welder, carpenter, skilled worker, electrician etc.,
**HH: Landholder and household
***MSM: Men who have sex with men
†CSW: Commercial sex workers; PPTCT: Prevention of parent to child transmission
First Line ART regimes in patients of immunological failure.
| Regimens | Number of patients (n) | Percentage (%) |
|---|
| ZLN | 190 | 41.49 |
| ZLE | 29 | 6.33 |
| SLN | 154 | 33.62 |
| SLE | 32 | 6.99 |
| OTHERS (TLN, TLE) | 53 | 11.57 |
ZLN: Zidovudine+Lamivudine+Nevirapine; ZLE: Zidovudine+Lamivudine+Efavirenz; SLN: Stavudine+Lamivudine+Nevirapine; SLE: Stavudine+Lamivudine+Efavirenz; TLN: Tenofovir+Lamivudine+Nevirapine; TLE: Tenofovir+Lamivdine+Efavirenz
Baseline CD4 counts distribution in patients of immunological failure.
| Baseline CD4 range (cells/mm3) | Male (%) (n=331) | Female (%) (n=127) |
|---|
| 1-50 | 61 (18.43) | 10 (7.87) |
| 51-100 | 70 (21.15) | 16 (12.60) |
| 101-150 | 43 (12.99) | 22 (17.32) |
| 151-200 | 48 (14.50) | 24 (18.90) |
| 201-250 | 27 (8.16) | 19 (14.96) |
| 250-300 | 24 (7.25) | 9 (7.09) |
| >300 | 58 (17.52) | 27 (21.26) |
p-value=0.008 (p-value<0.05 considered significant)
Associated conditions in patients of immunological failure.
| Associated conditions | Number of patients (n) | Percentage (%) |
|---|
| Tuberculosis | 101 | 22.05 |
| Extra pulmonary tuberculosis | 20 | 4.36 |
| Candidiasis | 66 | 14.41 |
| Herpes | 19 | 4.15 |
| Diarrhoea (Microspora, Isospora, Cryptosporidium complex) | 56 | 12.23 |
| Pneumocystis pneumonia | 8 | 1.75 |
| Anaemia | 69 | 15.07 |
| Others | 119 | 25.98 |
Plasma Viral Load (PVL) values in patients of immunological failure.
| Plasma viral load (copies/mL) | Number of patients (n=303) | Percentage (%) |
|---|
| Undetected (UD) and low viral load (<1000) | 54 | 17.82 |
| High viral load (>1000) | 249 | 82.18 |
Predictors of CD4 response of more than 100 cells decrease, 6 months after HAART.
| Last CD4 count before HAART (cells/mm3) | OR (95% CI) | p-value |
|---|
| 0-200 | 1.39 (0.37-4.39) | 0.5463 |
| *201-350 | 1.00 | - |
| 351-500 | 0.33 (0.11-1.12) | 0.0267 |
| >500 | 0.17 (0.05-0.69) | 0.0007 |
*Reference category
p-value <0.05- significant
Comparison of immunological failure cases with changing ART initiation guidelines.
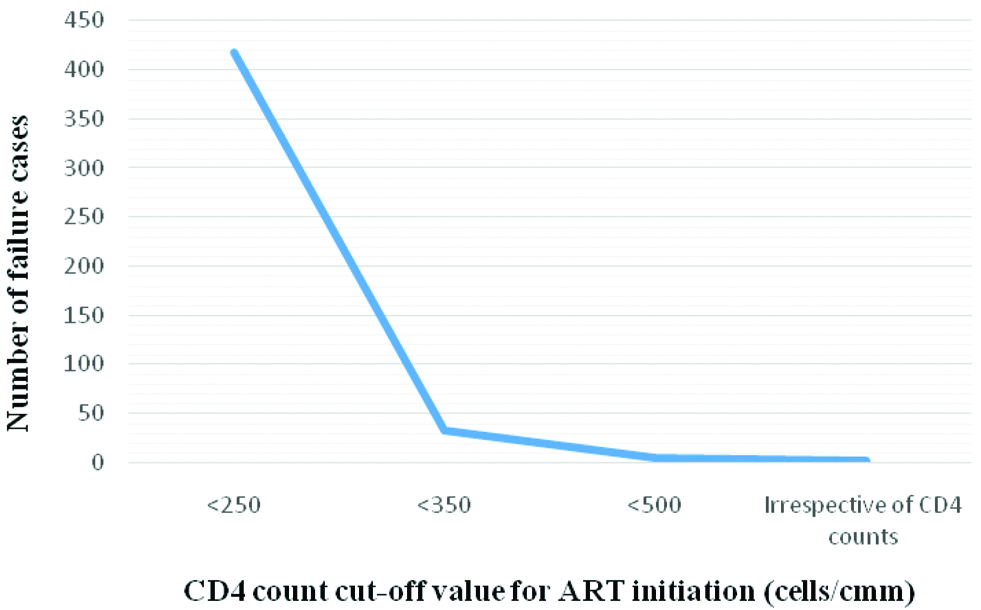
Year wise percentage of patients of immunological failure.
| Year | No. of patients on ART | No. of patients of immunological failure | Percentage % |
|---|
| 2012 | 772 | 37 | 4.79 |
| 2013 | 734 | 99 | 13.48 |
| 2014 | 750 | 97 | 12.93 |
| 2015 | 729 | 108 | 14.81 |
| 2016 | 911 | 65 | 7.14 |
| 2017 | 1195 | 52 | 4.35 |
| TOTAL | 5091 | 458 | 8.99 |
Discussion
Initiation of ART should help to achieve immune recovery and PVL suppression [7]. However, immunological failure despite complete viral suppression which is referred to as “immunological discordance”, were seen in some patients [9]. Other patients exhibit a different pattern of discordant response, characterised by a sustained CD4+ cell count response, despite persistent viremia [10].
CD4 count and PVL are used for patient management and as prognostic markers of disease progression and treatment outcomes [8]. Though PVL is considered to be the gold standard for evaluating treatment, it is often limited by cost. Therefore, in resource-limited settings, monitoring of CD4 counts holds more value.
Most patients in the study group (92.79%) were less than 54 years of age. Mean age reported by Agarwal A et al., was 38.10±7.89 years whereas Prabhakar B et al., observed the median age to be 38.5 years [15,16]. Gesesew HA et al., observed that age between 25-<50 years was associated with immunological failure among adult patients [22]. Heterosexual route was the most common mode of transmission in the study population (82.10%). Karade SK et al., reported heterosexual route of transmission in 90.4% patients and Kyaw NTT et al., observed this in 68% patients [23,24]. Studies have reported men to be more vulnerable to failure than women [15,25-27]. This has been explained by the fact that females are usually detected to be HIV positive after their husbands are detected reactive for HIV. Thus, their therapy is started at an earlier clinical stage and hence they tend to respond better [17,28]. In this study, significant percentage of males were found to have low (<100) baseline CD4 counts. Further, risk of this low baseline counts was found to be almost three times higher in males as compared to females. (OR=2.81 95% CI 1.68-4.83, p=0.0001).
Zidovudine is a common drug used in first line ART regimens [3]. The use of Zidovudine is associated with inadequate immune CD4 recovery [8,20,29]. The reasons are poorly understood; the probable explanation is related to the bone marrow suppression caused by use of zidovudine [29]. In this study, 47.82% patients showed the use of Zidovudine.
Tuberculosis is the most commonly detected serious opportunistic infection among PLHIV in India [3]. Other common opportunistic infections in HIV patients include candidiasis, Pneumocystis carinii pneumonia, herpes, cytomegalovirus disease etc., [30,31]. The most common co-existing conditions in this study were tuberculosis and anaemia. The onset of tuberculosis in HIV patients causes release of pro-inflammatory cytokines which cause activation of lymphocytes and macrophages resulting in increased viral load [32]. In the context of second-line ART, drug-drug interactions with anti-TB drugs also have to be considered [3]. Various authors have described tuberculosis and prolonged diarrhoea as risk factors for failure [15,19,27,33].
It has been observed that patients of immunological failure have low baseline CD4 counts (<350 cell/mm3) and this has been attributed to be a risk factor for failure [15-17,22,33-35]. Similar results were observed in this study (OR-1.39). The rate of discordance in this study was 17.82%. Moore DM et al., reported a VL+/CD4- discordant response in 15.4% patients [36]. The discordance rate as observed by Piketty C et al., was 10.5% and that by Grabar S et al., was 17.3% [11,12]. The pathogenesis of discordant responses is thought to be an interplay between various host, viral and treatment related factors such as age, low baseline CD4 cell counts, thymic involution, genetic polymorphisms, Zidovudine use, poor adherence to therapy etc., [10,16,20]. Estimation of PVL done in patients of immunological failure can help to identify these discordant responses and avoid unnecessary switching to second line ART [16,17,37].
The 90-90-90 target set by the UNAIDS is an ambitious treatment target to help end the AIDS epidemic. The results of India according to the UNAIDS 2017 indicate that 79% of PLHIV knew their status and 56% of people living with HIV were on treatment. The results of this study, report the rate of immunological discordance in patients which should always be kept in mind by clinicians during treatment of HIV patients since it affects their outcomes. Hence, this knowledge will help us to reach closer to the 90-90-90 target. In addition, estimation of PVL will help in the quantification of the third 90 goal of this target.
A decreasing trend was seen in the number of patients of failure when ART was started at higher CD4 counts [Table/Fig-7]. This may be due to coinciding of change in ART initiation guidelines [3-6] and time period of the study. However, it suggests that early initiation of ART can lead to a better prognosis as recommended by WHO [7].
In this study, the phenomenon of ‘discordance’ can be seen in patients on ART. The important data of immunological failure cases from this centre representing Central India which will help in monitoring the outcome of National AIDS Control Programme Phase-IV (NACP-IV).
Limitation(s)
The PVL of all patients was not available, as the testing was not done at our centre. Thus, CD4 counts remained the mainstay for monitoring the patients. In addition, it was a retrospective study and relied on the records of the patients.
Conclusion(s)
The phenomenon of ‘discordance’ can be seen in patients on ART. A discordance rate of 17.82% suggests the importance of PVL assay before switching to second line ART. Awareness and vigilance about this phenomenon is necessary to prevent unnecessary switching of regimen. It will also lead to improved patient outcomes and hence be helpful to reach closer to the 90-90-90 target. Local data collected and analysed will help the clinicians working with HIV-AIDS patients to contribute towards better management of these patients especially the ones with immunological failure.
*Others: welder, carpenter, skilled worker, electrician etc.,
**HH: Landholder and household
***MSM: Men who have sex with men
†CSW: Commercial sex workers; PPTCT: Prevention of parent to child transmission
ZLN: Zidovudine+Lamivudine+Nevirapine; ZLE: Zidovudine+Lamivudine+Efavirenz; SLN: Stavudine+Lamivudine+Nevirapine; SLE: Stavudine+Lamivudine+Efavirenz; TLN: Tenofovir+Lamivudine+Nevirapine; TLE: Tenofovir+Lamivdine+Efavirenz
p-value=0.008 (p-value<0.05 considered significant)
*Reference category
p-value <0.05- significant
Author Declaration:
Financial or Other Competing Interests: As declared above
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 03, 2020
Manual Googling: May 08, 2020
iThenticate Software: May 25, 2020 (10%)
[1]. National Operational Guidelines for Viral Load Testing 2018, NACO, Ministry of Health and Family Welfare, Government of India http://naco.gov.in/sites/default/files/National%20Operational%20Guidelines%20for%20Viral%20Load%20Testing%20Mar%2718.pdf [Google Scholar]
[2]. Sharma R, Pai C, Kar H, A retrospective analysis of discordant CD4 and viral load responses in HIV patients on anti-retroviral therapyInternational Journal of Scientific and Research Publications 2013 3(1):2011-13. [Google Scholar]
[3]. National AIDS Control Organisation. (2013). Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents: May 2013. http://naco.gov.in/sites/default/files/Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents%20May%202013%281%29_0.pdf [Google Scholar]
[4]. Antiretroviral Therapy Guideline for HIV-infected Adults and Adolescents including Post-exposure Prophylaxis, May 2007, NACO, Ministry of Health And Family Welfare, Government of India, accessed on June 21, 2018 https://apps.who.int/medicinedocs/documents/s18021en/s18021en.pdf [Google Scholar]
[5]. Office Memorandum No. T-11020/86/2006-NACO (ART), Ministry of Health and Family Welfare, Government of India, National AIDS Control Organization, dated 24 June 2016. http://www.naco.gov.in/sites/default/files/OMonrevisionofARTinitiationGuidelines2016.pdf [Google Scholar]
[6]. Office Memorandum No. T-11020/86/2006-NACO (ART), Ministry of Health and Family Welfare, Government of India, National AIDS Control Organization, dated 5 May 2017. http://naco.gov.in/sites/default/files/Scan_OM%20CST.pdf [Google Scholar]
[7]. World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach- Second edition. http://www.who.int/hiv/pub/arv/arv-2016/en/ [Google Scholar]
[8]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf, accessed on August 19, 2018 [Google Scholar]
[9]. Jevtovic D, Salemovic D, Ranin J, Pesic I, Zerjav S, Djurkovic-Djakociv O, The dissociation between virological and immunological responses to HAARTBiomedicine & Pharmacotherapy 2005 59(8):446-51.10.1016/j.biopha.2005.07.00616140494 [Google Scholar] [CrossRef] [PubMed]
[10]. Mauro S, Suely HT, Discordant immunological and virological responses to antiretroviral therapyJournal of Antimicrobial Chemotherapy 2006 58(3):506-10.10.1093/jac/dkl26316854959 [Google Scholar] [CrossRef] [PubMed]
[11]. Piketty C, Castiel P, Belec L, Batisse D, Mohamed AS, Gilquin J, Discrepant responses to triple combination antiretroviral therapy in advanced HIV diseaseAIDS 1998 12(7):745-50.10.1097/00002030-199807000-000119619806 [Google Scholar] [CrossRef] [PubMed]
[12]. Grabar S, Moing VL, Goujard C, Leport C, Kazatchkine MD, Costagliola D, Clinical outcome of patients with HIV-1 Infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapyAnnals of Internal Medicine 2000 133(6):401-10.10.7326/0003-4819-133-6-200009190-0000710975957 [Google Scholar] [CrossRef] [PubMed]
[13]. 90-90-90 An ambitious treatment target to help end the AIDS epidemic, UNAIDS/JC2684 (English original, October 2014), accessed on August 17, 2018. http://www.unaids.org/en/resources/documents/2014/90-90-90 [Google Scholar]
[14]. UNAIDS. Countries: India overview. [Internet] Geneva. Available from: https://www.unaids.org/en/regionscountries/countries/india [Google Scholar]
[15]. Agarwal A, Singh A, Chakravarty J, Sundar S, Rai M, Predictive markers of failure of first line anti-retroviral treatment in HIV patients in IndiaJournal of AIDS & Clinical Research 2013 4(5):210-15. [Google Scholar]
[16]. Prabhakar B, Banu A, Pavithra HB, Chandrashekhara P, Sasthri S, Immunological failure despite virological suppression in HIV seropositive individuals on antiretroviral therapyIndian Journal of Sexually Transmitted Diseases 2011 32(2):94-98.10.4103/0253-7184.8541222021970 [Google Scholar] [CrossRef] [PubMed]
[17]. Ingole N, Mehta P, Pazare A, Paranjpe S, Sarkate P, Performance of immunological response in predicting virological failureAIDS Research and Human Retroviruses 2013 29(3):541-46.10.1089/aid.2012.026623137294 [Google Scholar] [CrossRef] [PubMed]
[18]. Anusuya GS, Chockalingam C, Gurusamy M, Nadol P, Krishnaraj R, Radhakrishnan E, Various immunologic and virologic responses to second line antiretroviral therapy in Tambaram, IndiaJournal of AIDS and Clinical Research 2016 7(8):601-05.10.4172/2155-6113.1000601 [Google Scholar] [CrossRef]
[19]. Sadashiv MS, Rupali P, Manesh A, Kannangai R, Abraham OC, Pulimood SA, Risk factors of clinical and immunological failure in south Indian cohort on generic antiretroviral therapyThe Journal of the Association of Physicians of India 2017 65(12):03-08. [Google Scholar]
[20]. Kumar RS, Immunovirological discordance in HIV. In: Kamath S, Nadkar MY, editorsMedicine Update 2012 IndiaJaypee Brothers Medical Publishers:89-93. [Google Scholar]
[21]. Dean AG, Sullivan KM, Soe MM, OpenEpi: Open Source Epidemiologic Statistics for Public Health, Versionwww.OpenEpi.com, updated 2013/04/06 [Google Scholar]
[22]. Gesesew HA, Ward P, Woldemichael K, Mwanri L, Immunological failure in HIV infected adults from 2003 to 2015 in Southwest Ethiopia: A retrospective cohort studyBMJ Open 2018 8(8):e01741310.1136/bmjopen-2017-01741327631260 [Google Scholar] [CrossRef] [PubMed]
[23]. Karade SK, Ghate MV, Chaturbhuj DN, Kadam DB, Shankar S, Gaikwad N, Cross-sectional study of virological failure and multinucleoside reverse transcriptase inhibitor resistance at 12 months of antiretroviral therapy in Western IndiaMedicine 2016 95(37):37-47. [Google Scholar]
[24]. Kyaw NTT, Kumar AMV, Oo MM, Oo HN, Kyaw KWY, Thiha S, Long-term outcomes of second-line antiretroviral treatment in an adult and adolescent cohort in MyanmarGlobal Health Action 2017 10(1):129091610.1080/16549716.2017.129091628594295 [Google Scholar] [CrossRef] [PubMed]
[25]. Vanobberghen FM, Kilama B, Wringe A, Ramadhani A, Zaba B, Mmbando D, Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: Analysis of routinely collected national dataTropical Medicine and International Health 2015 20(7):890-92.10.1111/tmi.1250725779383 [Google Scholar] [CrossRef] [PubMed]
[26]. Penot P, Héma A, Bado G, Kaboré F, Soré I, Sombié D, The vulnerability of men to virologic failure during antiretroviral therapy in a public routine clinic in Burkina FasoJournal of the International AIDS Society 2014 17:1864610.7448/IAS.17.1.1864624433983 [Google Scholar] [CrossRef] [PubMed]
[27]. Hailu GG, Hagos DG, Hagos AK, Wasihun AG, Dejene TA, Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern EthiopiaPLoSONE 2018 13(5):e019625910.1371/journal.pone.019625929715323 [Google Scholar] [CrossRef] [PubMed]
[28]. Kumarasamy N, Venkatesh KK, Cecilia AJ, Devaleenol B, Saghayam S, Yepthomi T, Gender-based differences in treatment and outcome among HIV patients in south IndiaJournal of Women’s Health 2008 17(9):1471-75.10.1089/jwh.2007.067018954236 [Google Scholar] [CrossRef] [PubMed]
[29]. Wandeler G, Gsponer T, Mulenga L, Garone D, Maskew M, Prozesky H, Collaborative analysis of cohort studies in Southern AfricaAIDS 2013 27(14)10.1097/QAD.0b013e328362d88723660577 [Google Scholar] [CrossRef] [PubMed]
[30]. Shafran SD, Opportunistic infections in HIV-infected patientsCanadian Journal of Infectious Diseases and Medical Microbiology 1992 3(2):82-87.10.1155/1992/41371322529738 [Google Scholar] [CrossRef] [PubMed]
[31]. Khan AP, Malik A, Khan SH, Profile of candidiasis in HIV infected patientsIranian Journal of Microbiology 2012 4(4):204-09. [Google Scholar]
[32]. Orenstein MJ, Fox C, Wahl SM, Macrophages as a source of HIV during opportunistic infectionsScience 1997 276(5320):1857-61.10.1126/science.276.5320.18579188531 [Google Scholar] [CrossRef] [PubMed]
[33]. Rajian M, Gill PS, Chaudhary U, Prevalance of virological failure amongst WHO-defined immunological failure HIV patients on first line of highly active antiretroviral therapy in a tertiary care hospital in Haryana, IndiaInternational Journal of Research in Medical Sciences 2016 4(5):1613-19.10.18203/2320-6012.ijrms20161236 [Google Scholar] [CrossRef]
[34]. Teshome W, Tefera A, Detection of immunological treatment failure among HIV infected patients in Ethiopia: A retrospective cohort studyBMC Immunology 2015 16:5510.1186/s12865-015-0120-126376828 [Google Scholar] [CrossRef] [PubMed]
[35]. Bayou B, Sisay A, Kumie A, Assessment of the magnitude and associated factors of immunological failure among adult and adolescent HIV-infected patients in St. Luke and Tulubolo Hospital, Oromia Region, EthiopiaThe Pan African Medical Journal 2015 21:29110.11604/pamj.2015.21.291.683126587140 [Google Scholar] [CrossRef] [PubMed]
[36]. Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapyJournal of Acquired Immunodeficiency Syndrome 2005 40(3):288-93.10.1097/01.qai.0000182847.38098.d116249702 [Google Scholar] [CrossRef] [PubMed]
[37]. Kanapathipillai R, McGuire M, Mogha R, Szumilin E, Heinzelmann A, Pujades-Rodríguez M, Benefit of viral load testing for confirmation of immunological failure in HIV patients treated in rural MalawiTropical Medicine & International Health 2011 16(12):1495-500.10.1111/j.1365-3156.2011.02874.x21883726 [Google Scholar] [CrossRef] [PubMed]