Severe COVID-19 Infection While on Long Standing Therapy with Hydroxychloroquine
Kartik Ramakrishna1, Gaston Habib2, Japjot Chahal3, Dragos Manta4, Pratishtha Gupta5
1 Assistant Professor, Department of Pulmonary and Critical Care Medicine, State University of New York, Syracuse, New York, USA.
2 Resident, Department of Internal Medicine, State University of New York, Syracuse, New York, USA.
3 Resident, Department of Internal Medicine, State University of New York, Syracuse, New York, USA.
4 Assistant Professor, Department of Pulmonary and Critical Care Medicine, State University of New York, Syracuse, New York, USA.
5 Assistant Professor, Department of Internal Medicine, State University of New York, Syracuse, New York, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Kartik Ramakrishna, 750 E, Adams St, Syracuse, New York, USA.
E-mail: kartik_ramakrishna@hotmail.com
The Coronavirus Disease 2019 (COVID-19) pandemic has placed a large demand on healthcare resources worldwide. Hydroxychloroquine is reported as an agent with potential benefit in prophylaxis and treatment and is being used for both. The authors hereby present the case of 63-year-old male patient with myasthenia gravis and 22-year-old female patient with mixed connective tissue disease, both on chronic therapy with hydroxychloroquine, who were admitted to intensive care with severe Acute Respiratory Distress Syndrome (ARDS) and COVID-19 infection. The patients had typical symptoms of fever, cough and dyspnea with lymphopenia on blood investigations. They were intubated and mechanically ventilated, requiring paralytics and proning, with subsequent dramatic improvement in oxygenation noted. This is the first report to describe the development of SARS-CoV-2 infection in patients already on hydroxychloroquine and demonstrates the need for robust clinical studies before we can recommend its use for routine prophylaxis in healthcare workers or exposed patient contacts.
Anti-malarial drug, Coronavirus disease, Covid, Pandemic, Prophylaxis
Case Report
Case 1
The patient is a 63-year-old male with myasthenia gravis diagnosed in 2014, being treated with Hydroxychloroquine (HCQ) 200 mg twice daily, pyridostigmine and monthly Intra Venous Immunoglobulin (IVIG). Additional history includes thymoma with pulmonary metastasis with left upper and lower lobe wedge resections and thymectomy in 2015, pheochromocytoma and left adrenalectomy, type 2 diabetes, asthma, and atrial flutter. The patient presented to the emergency room with fever, cough, dyspnea and myalgia for three days. He was tachypneic, tachycardic and hypoxemic with a non-rebreather mask.
Initial laboratory studies showed lymphopenia (absolute lymphocytes 0.23×103/μL of blood), impaired renal function (creatinine 1.64 mg/dL, BUN 30 mg/dL), hyperglycaemia (194 mg/dL), elevated procalcitonin (5.22 ng/mL) and elevated lactate (3.9 mmol/L). Chest X-ray had bilateral lung infiltrates without pleural effusions. Respiratory viral panel was negative. PCR for SARS-CoV-2 was eventually positive.
He was intubated and admitted to the Intensive Care Unit (ICU) with ARDS. Mechanical ventilation in keeping with ARDS net recommendations was initiated with initial P/F ratio: 162. Home therapy of pyridostigmine and HCQ was continued, along with broad spectrum antibiotics for possible secondary bacterial infection. His P/F ratio worsened to 126 but improved to 352 after 16 hours of initiation of proning and paralytic therapy. On discontinuation of proning, PF ratio declined to 148 and proning was resumed without paralytics. At the time of writing this, the patient remains in ARDS and is undergoing proning daily.
Case 2
The patient is a 22-year-old female with a history of mixed connective tissue disorder and Raynaud’s phenomenon diagnosed at age 16, treated with HCQ 200 mg twice daily and Methotrexate 20 mg weekly. Pulmonary function testing was normal four months prior to presentation. She initially presented to her primary care physician with dyspnea, sore throat, cough and fever. She was diagnosed with Influenza and given Oseltamivir. Over the next three weeks, her dyspnea worsened and she remained febrile. Testing for SARS-CoV-2 virus was positive. Due to the severity of her symptoms, she presented to the hospital.
At presentation, she was febrile at 38.9°C and tachycardic (138/min), with oxygen saturation >98% on room air. Blood tests lymphopenia (absolute lymphocyte count 0.52×10/μL). Lactate dehydrogenase was 249 U/L, ferritin 145 ng/mL, D-dimer 0.49 mcg/mL, procalcitonin 0.12 ng/mL, C reactive protein 56.8 mg/L and erythrocyte sedimentation rate 18 mm/hr. Respiratory viral panel was negative. Chest X-ray showed early alveolar infiltrates predominantly in the right middle lobe [Table/Fig-1].
On the left is the X-ray obtained at admission, with early infiltrates in the right middle lobe. On the right is the X-ray obtained on day 3, showing dense consolidation with air bronchograms in the right middle and lower lobes with early infiltrates in the left lower lobe and lingula.
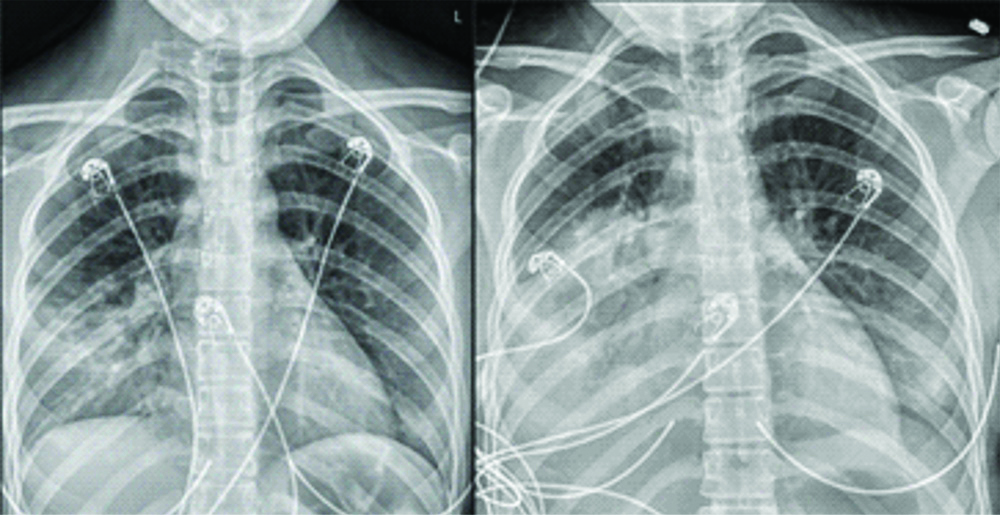
The patient was admitted to the ICU, where she remained febrile (40.2°C) and tachypneic. Empiric antibiotic therapy with Vancomycin and Piperacillin-Tazobactum was started. Blood and sputum cultures remained without growth and antibiotics were discontinued. Methotrexate was held. In discussion with Infectious Disease Department, HCQ was held due to the paucity of data to support its use. The following day she developed acute hypoxic respiratory failure with SpO2 87%, requiring oxygen via nasal cannula at 6 L/min. On day three, she continued to remain febrile. Chest X-ray showed lobar consolidation of the right middle, right lower and left lower lobes. Hypoxia continued to worsen eventually necessitating intubation. Arterial blood gas was consistent with severe ARDS with P/F ratio 62. Low tidal volume mechanical ventilation was initiated. Due to difficulty in oxygenating, she was placed on Airway Pressure Release Ventilation (APRV). The decision was made to initiate proning and paralytic therapy. On day four, repeat cell counts showed persistent lymphopenia. The ferritin, D-dimer, and C reactive protein value had increased to 843 ng/mL, 3.24 mcg/mL and 233.5 mg/L, respectively. Based on experience from Wuhan, China showing decreased oxygen requirements and resolution of fever, IV Methylprednisolone 1 mg/kg twice daily was started. Extracorporeal Membrane Oxygenation (ECMO) was considered, however her PF ratio had improved to 254 in prone position. At this time, she remains in intensive care, but improved to the point of extubation.
Discussion
On March 12th, 2020, the World Health Organization declared the outbreak of severe acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections a pandemic [1]. As of March 30th, there were 803,650 cases reported with 39,033 deaths [2]. Approximately, 5 to 10% of patients require admission to ICU [3]. HCQ gained attention based on reports of in-vitro activity and anecdotal reports. In March, HCQ was announced as a potential treatment in America. This has led to its extensive use worldwide. HCQ is even being used for prophylaxis in health workers in Asia. A national medical body in India issued a recommendation for its use for prophylaxis in healthcare-workers and household contacts of patients [4]. Subsequently, there is a shortage of HCQ for patients with proven indications. In the cohort from China, 10% had history of cancer, none were on immunosuppressant therapy [5].
Both patients presented with fever, dry cough and dyspnea, with lymphopenia, similar to descriptions from Bhatraju PK et al., of critically ill patients admitted to ICU in Seattle and descriptions from Wang D et al., of hospitalised patients at Wuhan, China [6,7]. The majority of patients in both cohorts had co-morbid conditions, diabetes and hypertension being most common. Both the index patients had significant co-morbid conditions requiring immunomodulation and longstanding therapy with HCQ. In a cohort study from Seattle, 3 had received steroids for presumed exacerbation of asthma, and one had history of HIV infection (CD4 count and viral load not reported) [6]. To the best of our knowledge, this is the first report of patients on chronic therapy with HCQ to develop this condition.
Chloroquine and HCQ were both reported to have in-vitro benefit in SARS-CoV-2 activity [8,9]. Chloroquine was reported in a letter to be an efficacious drug in improving the disease course in over 100 patients, however no further data was provided [10]. Open label non-randomised data of combination Azithromycin and HCQ of 36 hospitalised patients showed that the treatment group were more likely to test negative for virus on day 6 [11].
Evidence for use of HCQ for prophylaxis does not exist. A clinical trial of HCQ for post-exposure prophylaxis has now been registered in the US.
Conclusion(s)
Both the patients had life threatening SARS-CoV-2 infection despite long term HCQ use. These reports are presented to serve as a reminder that more robust data is needed before HCQ can be recommended for prophylaxis or treatment of this disease.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 17, 2020
Manual Googling: Apr 27, 2020
iThenticate Software: May 22, 2020 (6%)
[1]. WHO Director-General’s opening remarks at the media briefing on COVID-19- 11 March 2020. [https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020] [Google Scholar]
[2]. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins CSSE. Retrieved 30 March 2020 [Google Scholar]
[3]. Wu Z, McGoogan JM, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and PreventionJAMA 2020 Feb 24 10.1001/jama.2020.264832091533 [Google Scholar] [CrossRef] [PubMed]
[4]. https://www.mohfw.gov.in/pdf/AdvisoryontheuseofHydroxychloroquinasprophylaxisforSARSCoV2infection.pdf [Google Scholar]
[5]. Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: Single-center experience from Wuhan, ChinamedRxivPreprint posted March 12, 202010.1101/2020.03.06.20032342 [Google Scholar] [CrossRef]
[6]. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Covid-19 in critically ill patients in the seattle region- Case seriesN Engl J Med 2020 Mar 30 10.1056/NEJMoa200450032227758 [Google Scholar] [CrossRef] [PubMed]
[7]. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-Infected pneumonia in Wuhan, ChinaJAMA 2020 323(11):1061-69.10.1001/jama.2020.158532031570 [Google Scholar] [CrossRef] [PubMed]
[8]. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitroCell Discov 2020 6:1610.1038/s41421-020-0156-032194981 [Google Scholar] [CrossRef] [PubMed]
[9]. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2)Clin Infect Dis 2020 Mar 9 :pii: ciaa23710.1093/cid/ciaa23732150618 [Google Scholar] [CrossRef] [PubMed]
[10]. Gao J, Tian Z, Yang X, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studiesBiosci Trends 2020 14(1):72-73.10.5582/bst.2020.0104732074550 [Google Scholar] [CrossRef] [PubMed]
[11]. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trialInt J Antimicrob Agents 2020 Mar 20 :10594910.1016/j.ijantimicag.2020.10594932205204 [Google Scholar] [CrossRef] [PubMed]