Adenomyoepithelioma (AME) is an uncommon tumour of breast, which is common in middle aged and older adults (>60 years). It is characterised by dual differentiation of cells into luminal as well as myoepithelial cells. Herein we report a case of a 22-year-old female who presented with a solitary swelling in the upper outer quadrant of the left breast, diagnosed as fibrocystic breast disease on Fine Needle Aspiration Cytology (FNAC). Excisional biopsy and histopathologic examination showed features suggestive of AME, which was confirmed on Immunohistochemistry (IHC) staining. On retrospective review of the cytology slides, a biphasic appearance was discerned; the myoepithelial cells probably having been masked by the cystic degeneration. AMEs are generally benign. However, in view of substantial reports of local recurrences, malignant transformations and metastases, accurate distinction of this lesion is crucial. Here, the authors report this rare case, to emphasise the importance of considering this entity in the differential diagnosis of a focal solid lesion in the breast, and to underline the accompanying diagnostic pitfalls.
Case Report
A 22-year-old female presented with a solitary painless swelling in the left breast, gradually increasing in size since last 3 years. There was no other symptom or any relevant history. Local examination revealed a firm lump in the upper outer quadrant of the left breast measuring 2 cm × 1.5 cm. The nipple and overlying skin were normal. The lump was mobile, nontender, and not fixed to the underlying skin or muscle. There was no palpable lymphadenopathy. There was no nipple discharge or history of pregnancy or lactation. Hormone level estimation was not deemed necessary. An ultrasonographic evaluation of left breast revealed a well defined heteroechoic, lobulated lesion in the left breast measuring 1.87 cm × 1 cm. The radiological impression was fibroadenoma, and FNAC was advised.
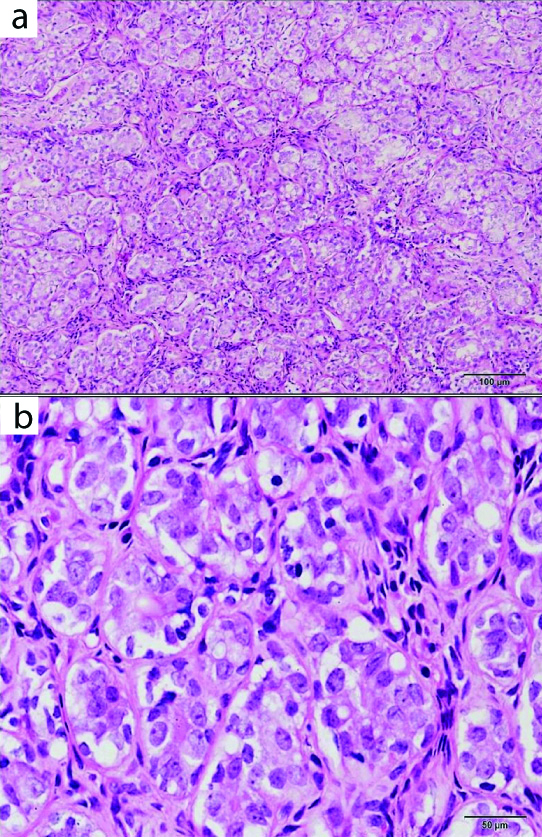
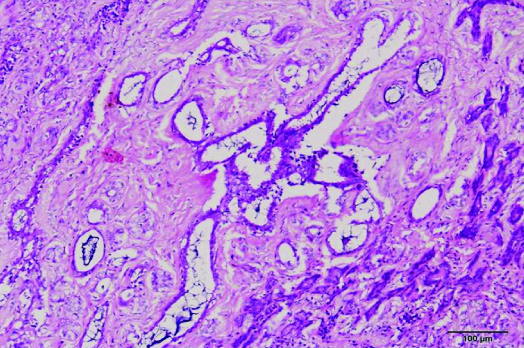
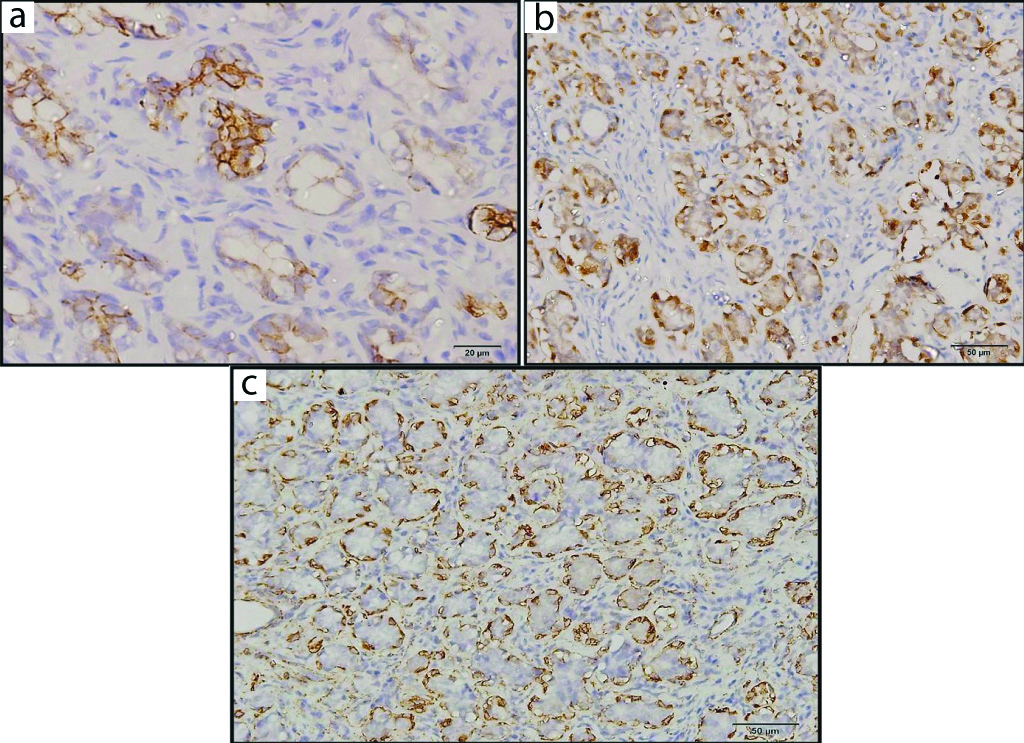
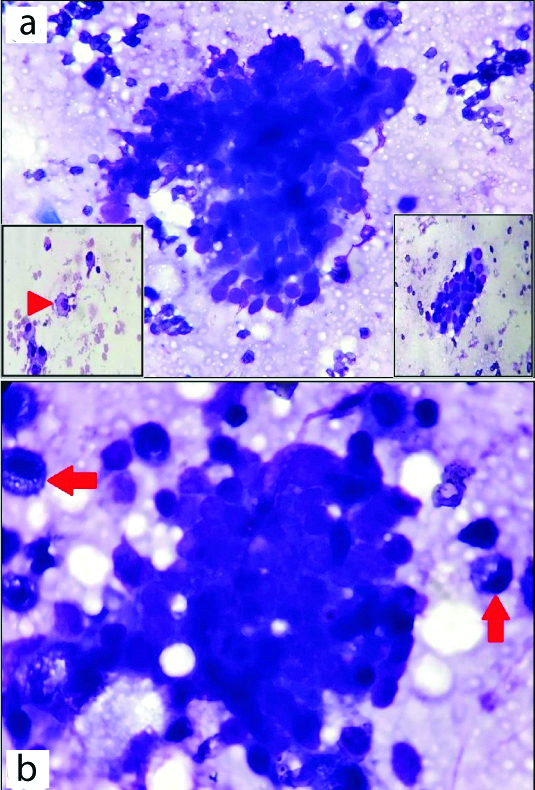
Pathological findings: FNAC yielded blood mixed cystic fluid, smears made from which showed moderate cellularity composed of predominantly sheets of cystic macrophages and a few clusters of benign ductal epithelial cells along with variable number of degenerated cells having vacuolated cytoplasm. Apocrine changes were also noted. Provisional diagnosis was suggestive of fibrocystic disease. Resection of the swelling was performed, as excision biopsy had been asked for. Gross examination showed a grey-white globular tissue measuring 2 cm × 1.5 cm × 1.5 cm, which on cut section was solid, homogenous and well circumscribed. Histopathological Examination (HPE) showed a well circumscribed mass composed of tubules of ductal epithelial cells surrounded by and admixed with variable population of cells exhibiting clear to plasmacytoid appearance [Table/Fig-1a,b]. These tubules were seen separated by thin fibrous tissue septa. Few areas also exhibited cystically dilated glands, apocrine changes and fibrous tissue [Table/Fig-2]. No atypia, necrosis and mitosis were identified. Tubular adenoma, fibroadenoma/fibrocystic disease and AME were at the forefront of the present differential diagnosis on HPE, as was also myoepithelioma although a little less likely. To unravel this quandary, IHC study was resorted to, which demonstrated positivity of the epithelial cells for CK7, while S100 and Smooth Muscle Actin (SMA) highlighted the myoepithelial cells surrounding the ductal cells [Table/Fig-3a-c]. Final diagnosis was reported as AME of the left breast. A retrospective review of the FNAC slides was done, as to ascertain the reasons for the misdiagnosis. Careful analysis now brought to light the fact that probably the cystic degeneration encountered on the cytology aspirates had masked the myoepithelial component. The degenerated cells having hyperchromatic nuclei and vacuolated cytoplasm reported in the aspirate could actually have been myoepithelial cells as well. Some intact viable myoepithelial cells were also found to have been intimately and tightly admixed within the clusters of ductal epithelial cells. Thus, a biphasic appearance was discerned, which had been missed in the initial approach [Table/Fig-4a,b]. As the tumour characteristics revealed a benign nature, no further treatment was necessary after the surgery; however, the patient was put on follow-up, which has been uneventful till date.
Histopathological examination of AME showed tubules of ductal epithelial cells surrounded by and admixed with variable population of cells exhibiting clear to plasmacytoid appearance; H&E, (a) X100; (b) X400.
Focal area showed cystically dilated glands and fibrous tissue. H&E, X100.
Immunohistochemistry underlined the epithelial cells positivity for CK 7 (a, X400), while S100 (b, X400) and SMA (c, X200) highlighted the myoepithelial cells surrounding the ductal cells.
a) FNAC smear showed cluster of ductal epithelial cells admixed with myoepithelial cells in a background of cyst macrophages (inset, red arrow heads); b) Degenerated cells exhibiting hyperchromatic nuclei with vacuolated cytoplasm were categorised as myoepithelial cells (arrows); {MGG stain, (a) and (insets) X400, (b) X1000}.
Discussion
Breast AME arises from a mixture of epithelial and myoepithelial cells and is a rare, myoepithelial cell-rich neoplasm. WHO has essentially categorised AME as a benign tumour where both the epithelial and myoepithelial component are histologically non-malignant, however, cautions about the fact that either of the two components can undergo malignant transformation (AME with carcinoma) [1,2]. AMEs usually occur in the fifth and sixth decades, are usually solitary, exceed 1 cm in size, and present as a well circumscribed mass lesion [2-4].
Cytologic diagnosis of AMEs can be difficult, owing to the varied histology and the rarity of the lesion. At Memorial Sloan-Kettering Cancer Center a retrospective evaluation of cytologic findings from 12 patients with histologically proven benign AMEs showed that none of the patients had been diagnosed originally as AME [5]. The cytological aspirates are typically cellular, which exhibit cohesive sheets of uniform ductal cells with interspersed myoepithelial cells and dispersed stripped spindle-shaped nuclei of myoepithelial cell origin. These traits of an AME, namely a bland bimodal cell population admixed with stromal elements and with a preponderance of myoepithelial cells are shared by fibroadenomas and tubular adenomas as well, and thus differentiating the three can be an arduous task [6,7]. Also, fibrocystic disease, papilloma and other myoepithelial/stromal cell-rich lesions such as phyllodes tumour, myoepithelioma, myofibroblastoma and even adenoid cystic carcinoma may mimic AMEs [7,8]. Myoepithelial cells in breast FNAC usually appear as small, comma shaped or ovoid, dark nuclei that are devoid of cytoplasm seen admixed with the clusters of ductal cells or scattered in the background as bipolar, naked nuclei. These cells can also take up an epithelioid, plasmacytoid, spindled or clear cell appearance, or can even manifest mixed cell morphologies. The myoepithelial component in the present sample was probably masked by the presence of cystic degeneration. Some cells having hyperchromatic nuclei and vacuolated cytoplasm which had been designated as degenerated cells could have been myoepithelial cells. It is pertinent to note that myoepithelial cells, apart from the myriad appearances which they can assume, can also seem like degenerated cells, as noted by Iyengar P et al., which could be a similar observation as in the present study. In addition to myoepithelial and epithelial cells, other cell types that can be encountered in FNAC of AME are foamy macrophages and apocrine cells. However, no feature alone and no features in combination should be regarded as specific or characteristic of AME [5].
Histologically, AME manifests a biphasic pattern characterised by proliferation of myoepithelial cells surrounding cuboidal or columnar-shaped epithelium-lined spaces. Depending upon the distribution of proliferating glandular and myoepithelial cell component, the extent of different configuration of myoepithelial cells such as spindle or polygonal, the prominence of papillary component and the degree of fibrosis, AMEs can unveil varied histomorphologic patterns. Tavassoli FA, using a mixture of architectural and cytological features, subdivided AME into three morphological patterns: lobulated, spindle cell, and tubular [9]. Most AMEs exhibit the tubular pattern, typified by proliferation of luminal glandular cells rimmed by outer layer of prominent myoepithelial cells of abundant clear cytoplasm [4,9], as was seen in the present case. The myoepithelial cells in AME are more numerous and larger in size as compared to cells of normal breast lobules, adenosis nodules or simple papillomas. Often, myoepithelial cells have clear glycogen rich cytoplasm, but myoepithelial cells with spindle shape are common. Sometimes the myoepithelial cells can also have abundant eosinophilic cytoplasm imparting a ‘myoid’ appearance and can simulate a leiomyoma. In other cases, the myoepithelial cells have a plasmacytoid appearance with eccentric nuclei and eosinophilic glassy cytoplasm and may overgrow the ductal elements to form sheets and irregular nests and cords [3].
Due to such varying degree of both architectural and cytological variations, a host of tumours enter the differential diagnosis of AMEs on histopathology too, with the spectrum ranging from epithelial tumours having a myoepithelial component to those lesions composed of a prominent myoepithelial/stromal cell-rich component. Fibroadenoma, tubular adenoma, microglandular adenosis, nipple adenoma and myoepithelioma were the other diagnostic possibilities considered in this case. The distinguishing features are discussed in [Table/Fig-1].
The epithelial component stains positive with low molecular weight keratins Cam 5.2, CK7, CK8/18, and EMA, while the myoepithelial cell component is highlighted by p63, SMA, calponin, Smooth Muscle Myosin Heavy Chains (SMMHC), S-100, CK5/6 and CK14 [1,3,4,10]. However, not infrequently, staining for the myoepithelial markers can be focal and variable. Thus, it is imperative to use more than one myoepithelial marker and to repeat the markers on additional blocks when the index of suspicion is high.
AMEs generally are benign neoplasms, but malignant transformation of either one or both components has been documented in rare tumours, and distant metastases to lung, brain and liver have been reported [11,12]. Because of its diversified morphology, AME remains an arduous diagnosis both on FNAC and needle biopsy. Veracious recognition of this entity is usually attainable only on excisional biopsy and IHC in conjunction with HPE plays an incredible role in demonstrating the biphasic nature of the tumour. Diligent and meticulous sampling of this tumour, owing to the morphologic heterogeneity as discussed above, is essential to rule out atypia, high mitotic activity or infiltrative margins, which may be indicators of malignancy [13]. This is vital as incomplete excision may lead to late recurrences. In the present case, there was no evidence of local invasion, high mitotic index and cytological atypia, hence proving the benign nature of AME. AMEs are generally cured by complete excision. There is no dictum for axillary lymph node dissection for these lesions, except in case of clinically involved nodes. Evidence is not in much favour of the role of radiotherapy or chemotherapy in the management of either AME or even AME with carcinoma [11,14].
Conclusion(s)
AME is a rare tumour of middle aged and older females, but it should always be entertained in the differential diagnosis of a solitary breast mass sampled by FNAC, even in young adults. Although the definitive diagnosis of AME may not always be possible on review of FNAC material alone, accurate identification of the myoepithelial cells is crucial. The above case illustrates the complexities in making a diagnosis of AME, and underscores the significance of flawlessly discerning such lesions and differentiating them from other benign proliferative diseases of the breast, since these cases need long term follow-up after wide local excision.
[1]. Korolczuk A, Amarowicz M, Bak K, Korobowicz E, Koncewicz T, Adenomyoepithelioma of the breast with late pulmonary metastases- Case report and review of the literatureJ Cardiothorac Surg 2016 11(1):121-26.10.1186/s13019-016-0518-827487934 [Google Scholar] [CrossRef] [PubMed]
[2]. Ito R, Ota D, Ando S, Mori M, Fukuuchi A, A case of adenomyoepithelioma with myoepithelial carcinoma of the breastClin Case Rep 2019 7(5):930-34.10.1002/ccr3.210031110717 [Google Scholar] [CrossRef] [PubMed]
[3]. Hayes MM, Adenomyoepithelioma of the breast: A review stressing its propensity for malignant transformationJ Clin Pathol 2011 64(6):477-84.10.1136/jcp.2010.08771821307156 [Google Scholar] [CrossRef] [PubMed]
[4]. Kim MJ, Kim CS, Ju MJ, Park YS, Malignant adenomyoepithelioma of the breast: A rare case reportIntl J Surg Case Reports 2019 59:111-14.10.1016/j.ijscr.2019.04.04531128547 [Google Scholar] [CrossRef] [PubMed]
[5]. Iyengar P, Ali SZ, Brogi E, Fine-needle aspiration cytology of mammary adenomyoepithelioma: A study of 12 patientsCancer 2006 108(4):250-56.10.1002/cncr.2183916544319 [Google Scholar] [CrossRef] [PubMed]
[6]. Mercado CL, Toth HK, Axelrod D, Cangiarella J, Fine-needle aspiration biopsy of benign adenomyoepithelioma of the breast: Radiologic and pathologic correlation in four casesDiagn Cytopathol 2007 35:690-94.10.1002/dc.2072217924402 [Google Scholar] [CrossRef] [PubMed]
[7]. Jassar A, Pathania K, Tubular variant of mammary adenomyoepithelioma: Diagnostic challenges and cytomorphological correlation in two casesCyto Journal 2017 14:2910.4103/cytojournal.cytojournal_26_1729333189 [Google Scholar] [CrossRef] [PubMed]
[8]. Satyanarayana V, Gole S, Adenomyoepithelioma A rare breast tumor: Case studies with review of the literatureThe Internet Journal of Pathology 2012 13(2):01-09.10.5580/2b55 [Google Scholar] [CrossRef]
[9]. Tavassoli FA, Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithe-lioma, and myoepithelial carcinomaAm J Surg Pathol 1991 15(6):554-68.10.1097/00000478-199106000-000041709559 [Google Scholar] [CrossRef] [PubMed]
[10]. Yoon JY, Chitale D, Adenomyoepithelioma of the breast: A brief diagnostic reviewArch Pathol Lab Med 2013 137(5):725-29.10.5858/arpa.2011-0404-RS23627458 [Google Scholar] [CrossRef] [PubMed]
[11]. Lakhani SR, Hayes M, Eusebi V, Adenomyoepithelioma and adenomyoepithelioma with carcinoma. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJWHO Classification of Tumours of the Breast 2012 4th edLyonIARC:122-3. [Google Scholar]
[12]. Kim SY, Huh S, Park HR, Oh MH, Adenomyoepithelioma of the Breast: Which Mimicking Malignancy on Ultrasound ElastographySoonchunhyang Med Sc 2019 25(1):57-61.10.15746/sms.19.009 [Google Scholar] [CrossRef]
[13]. Loose JH, Patchefsky AS, Hollander IJ, Lavin LS, Cooper HS, Katz SM, Adenomyoepithelioma of the breast. A spectrum of biologic behaviorAm J Surg Pathol 1992 16(9):868-76.10.1097/00000478-199209000-000051384377 [Google Scholar] [CrossRef] [PubMed]
[14]. Fang ZM, Tse RV, Marjoniemi VM, Kozlov S, Lavin MF, Chen H, Radioresistant malignant myoepithelioma of the breast with high level of ataxia telangiectasia mutated proteinJ Med Imaging Radiat Oncol 2009 53(2):234-39.10.1111/j.1754-9485.2009.02053.x19527373 [Google Scholar] [CrossRef] [PubMed]