Collection of fluid in the macula is called macular oedema and when the fovea is involved, it results in decreased visual acuity [1]. Macular oedema occurs in a large number of pathological conditions such as Age-Related Macular Degeneration (ARMD), diabetic retinopathy, hereditary retinal degenerations, Retinal Vein Occlusion (RVO), Choroidal Neovascular Membrane (CNVM), after cataract surgery, Epiretinal Membrane (ERM) and uveitis. The impact of non-communicable diseases is immense due to a rapidly growing geriatric population and this has led to a substantial increase in the number of patients suffering from these retinal disorders [2]. Hence, the clinical burden is enormous and this makes early detection as well as, effective management crucial to counteract the vision loss, caused by these diseases.
OCT is a non-contact, diagnostic method, which uses infrared light to analyse retinal structures by means of high- resolution tomographic cross-sections [3]. It is built on the principle of Michelson interferometry. OCT specialises in identification, localisation, quantification and long-term follow-up of retinal disorders. The Spectral or Fourier domain OCT (SD-OCT) has now replaced the older Time-domain OCTs. Hence, its advent has caused major improvements in technology and increased rapidity of diagnosis [4].
Till the advent of anti-VEGF, macular laser photocoagulation was practised as the gold standard treatment for macular oedema. The discovery of anti-VEGFs was a breakthrough in the management of macular oedema. Bevacizumab (Avastin, Genentech/Roche) is one such anti-VEGF, which targets all isoforms of VEGF. It was initially developed for chemotherapy of colorectal carcinoma [5].
In the current clinical scenario of a developing country like India, Bevacizumab (Avastin, Genentech/Roche) has emerged as a much cheaper option than its contemporaries Ranibizumab and Aflibercept. Comparative effectiveness research showed that IVB and Ranibizumab (IVR) had equal efficacy in treatments of Neovascular ARMD and Diabetic Macular Oedema (DME) [2]. Thus, subsequently, it gained widespread access as an off label medication in the field of ophthalmology for treatment of neovascular disorders [6].
IVB is associated with minimal complications, which include Intraocular Pressure (IOP) spikes, vitreous haemorrhage, endophthalmitis, anterior uveitis and tractional retinal detachment. Rare Serious Systemic Adverse Events (SSAEs) like hypertension, arterial thromboembolic events, venous thromboembolism, heart failure and vascular death, have been reported in few studies though never found statistically significant [2].
Single to multiple doses of the injection is usually required with or without other supportive treatments. Depending on the effect of one injection, it was decided about further requirement of more injections or other alternate therapy, if macular oedema fail to resolve.
This study was conducted with the aim to study the efficacy of IVB over a period of six months in patients with macular oedema secondary to various pathologies. Response to the IVB in the form of functional recovery along with, resolution of macular oedema on SD-OCT was studied. Intra and post-procedural, as well as drug-related side-effects, were noted down.
Materials and Methods
This was a retrospective observational longitudinal study done upon 32 eyes of 30 patients with macular oedema due to various retinal pathologies, who underwent IVB (Avastin) from January 2018 to April 2019 at a Tertiary care hospital in Western Maharashtra, India. Data from case record of patients were included and the saved OCT pictures were analysed.
Institutional Ethical Committee (IEC) approval {BV (DU)MC&H/Sangli/IEC/366/19} and written informed consent were obtained from the patient for this study, also separate consent was obtained for using the data for research purpose. The off-label use of the drug, its potential risks and benefits were clearly explained to the patients and their relatives.
Inclusion criteria: The study included data for patients 40 years and above, of both genders, having macular oedema due to different retinal disorders. Data were included only if there was a minimum follow-up of six months.
Exclusion criteria: The study excluded data for patients who had vitreous haemorrhage, macular oedema post cataract surgery (pseudophakic macular oedema) and patients who had already taken some other form of treatment before the injection such as Pan Retinal Photocoagulation (PRP) and vitrectomy.
The patients underwent Best Corrected Visual Acuity (BCVA) measurement using LogMAR chart; ophthalmic examination by using slit lamp biomicroscopy and IOP recorded using Goldmann Applanation tonometer.
Baseline central retinal characteristics were analysed using the OCT (Optovue) through a dilated pupil. The SD-OCT machine used for the study has 3D real time acquisition with 6 mm × 6 mm retina thickness map, 7 line high resolution Raster and 250 μ separation.
As per hospital protocol, culture sensitivity, gram positivity and potassium hydroxide (KOH) mount were carried out for every new vial of injection bevacizumab. All injections were performed under topical anaesthesia. Moxifloxacin eye drop was started 24 hours before the injection. A normal blood sugar report of respective patients was obtained before giving the injection. Under all sterile and aseptic conditions, first, the eye was anaesthetised using topical proparacaine hydrochloride 0.5% and then 5% Povidone-iodine solution was instilled in the conjunctival sac. Then 0.1 mL Bevacizumab was injected into the mid-vitreous, 3.5 mm and 3 mm posterior to the limbus in the temporal quadrant in phakic and pseudophakic eyes respectively, with a 1cc syringe and 32-gauge needle. Topical moxifloxacin eye drop was instilled four times daily for one month post-injection.
Patients were examined at baseline, one month, three month and six months after the injection by taking their OCT and BCVA.
Preparation of patient and technique for acquiring OCT (As per hospital protocol and case records): Dilating drops comprising of tropicamide 5% with phenylephrine 0.8% was used to dilate the pupil. The biodata of patients including name, date of birth and gender were fed into the OCT machine for normative database analysis. After pupils were dilated, the patient was seated in front of the OCT machine, the head resting on the support to keep it motionless. Then, the eye (OD/OS) (Oculus Dextrus/Oculus Sinister) and the type of scan, which was retina map in this case, were selected and the scanning started. The patient was instructed to look into the machine at the central green cross. Once the macula came into focus, the examiner clicked the capture button on the joystick. The OCT finally provided 7-line high resolution raster as well as 6 mm × 6 mm retinal thickness map. All seven OCT cuts were analysed and the largest measure of retinal thickness was recorded.
The retinal thickness map or ETDRS (Early Treatment of Diabetic Retinopathy Study) map divides the macula into nine regions with three concentric rings measuring 1 mm (innermost ring), 3 mm (inner ring) and 5 mm in diameter (outer ring) centred on the fovea. The innermost 1 mm ring is the fovea while the 3 mm inner ring and 5 mm outer ring is further divided into four equal regions (superior, inferior, nasal and temporal) representing the parafoveal and perifoveal area respectively.
The macular thickness of the central foveal zone (innermost ring) and the parafoveal zone (inner ring) were obtained. The inner ring measurement was taken as the average reading of the four quadrants.
After doing OCT, the changes in cystoid spaces, the changes in Hyper-Reflective Foci (HRF), changes in SMD height and regeneration of EZ were analysed at each subsequent visit and compared to the baseline.
During follow-up, a comprehensive ocular examination was done which included BCVA, IOP, anterior and posterior segment examinations. OCT was done in the above mentioned manner. Then change analysis was done which displayed the increase or decrease in macular oedema in the retinal thickness map and morphological changes in the raster line of the current follow-up, with respect to the baseline.
In cases of relapse and cases that did not attain expected decrease in macular thickness after first injection, reinjection was given. Relapse was suspected in those patients who showed increase in macular thickness on OCT and decrease in BCVA, after complete or partial resolution of Macular Oedema (ME) in previous follow-up visits.
Statistical Analysis
The paired t-test and chi-square tests were used for the comparison of data. For all statistical test, a p-value of <0.05 was considered statistically significant.
Results
During the study period, a total of 45 patients underwent IVB. Out of the 45 patients, 15 were excluded from the study for the following reasons: eight patients had follow-up data for less than six months, in four patients OCT analysis was not possible because of vitreous haemorrhage, two patients needed intravitreal injection in spite of previous photocoagulation and one patient in spite of previous vitrectomy, needed injection. Remaining 30 patients (32 eyes) fulfilled the inclusion criteria.
The mean age of the study population was 62.78±13.69 years (range: 40-85 years); of which 30% were females (9) and 70% were males (21) [Table/Fig-1]. Patients were followed-up for mean duration of 6.9±1.15 months (range 6-10 months).
Age and gender distribution of study patients.
| Age group in years | Number of patients (%) |
|---|
| 40-50 | 6 (20%) |
| 51-60 | 5 (16.66%) |
| 61-70 | 12 (40%) |
| 71-80 | 6 (20%) |
| 81-90 | 1 (3.33%) |
| Males | 21 (70%) |
| Females | 9 (30%) |
The most common indication for the injection is shown in [Table/Fig-2]. All patients underwent retinal OCT at baseline, one month, three months and six months.
Causes of macular oedema.
| Causes of macular oedema | Number of eyes (%) |
|---|
| Diabetic Macular Oedema (DME) | 16 (50%) |
| Retinal Vein Occlusion (RVO) | 10 (31.25%) |
| Choroidal Neo-vascular Membrane (CNVM) | 5 (15.62%) |
| Hypertensive retinopathy with macular oedema | 1 (3.12%) |
| Total | 32 (100%) |
The mean pre-injection 1 mm CFT was 445.28±155.87 μm at baseline that decreased to a mean of 334.44±100.42 μm at one month, at three months to 332.84±101.20 μm and at six months to 330.75±103.17 μm. The reduction in CFT noticed from baseline to one month post-injection was statistically significant (p<0.001). But further reduction in CFT from one month to three months and six months in present study was not statistically significant [Table/Fig-3].
Mean central foveal thickness at pre-operative, 1 month, 3 months, and 6 months.
| Time duration | Central foveal thickness Mean (±Standard deviation) |
|---|
| Pre-operative (Baseline) | 445.28 (±155.87) |
| 1 month | 334.44 (±100.42) (p<0.001) |
| 3 months | 332.84 (±101.20) (p=0.230) |
| 6 months | 330.75 (±103.17) (p=0.213) |
Similarly, the mean pre-injection 3 mm PFT was 452.70±131.95 μm at baseline that decreased to a mean of 353.09±100.92 μm at one month, at three months to 351.60±99.56 μm and at six months to 350.20±97.05 μm. The reduction in PFT noticed from baseline to one month post-injection was statistically significant (p<0.001). However, further reduction in PFT from one month to three months and six months was not statistically significant [Table/Fig-4].
Parafoveal thickness at pre-operative, 1 month, 3 months, and 6 months.
| Time duration | Parafoveal thickness Mean (±Standard deviation) |
|---|
| Pre-operative (Baseline) | 452.70 (±131.95) |
| 1 month | 353.09 (±100.92) (p<0.001) |
| 3 months | 351.60 (±99.56) (p=0.285) |
| 6 months | 350.20 (±97.05) (p=0.219) |
The improvement in BCVA was noted. At one month, the mean BCVA improved from 1.03 at baseline to 0.61, which was statistically significant (p<0.001). At three months and six months, the BCVA further improved to 0.43 and 0.31 respectively, both of which were statistically significant from the baseline (p<0.001) [Table/Fig-5].
Best corrected visual acuity (BCVA).
| Time duration | Mean BCVA (LogMar) |
|---|
| Pre-operative (Baseline) | 1.03 |
| 1 month | 0.61 (p<0.001) |
| 3 months | 0.43 (p<0.001) |
| 6 months | 0.31 (p<0.001) |
Morphological changes at baseline then at one, three and six months follow-up are mentioned in [Table/Fig-6].
| Number of eyes |
|---|
| Pre-operative | 1 month | 3 months | 6 months |
|---|
| A. Cystoid spaces: |
| 1 | 24 (100%) | | | |
| 0 | | 20 (83.33%) | 18 (75%) | 21 (87.5%) |
| 2 | | 4 (16.66%) | 6 (25%) | 3 (12.5%) |
| 1: Present; 0: Resolved; 2: Increased. |
| B. Hyper-reflective FOCI (HRF): |
| 1 | 15 (100%) | | | |
| 0 | | 13 (86.66%) | 13 (86.66%) | 15 (100%) |
| 2 | | 2 (13.33%) | 2 (13.33%) | |
| 1: Present; 0: Decreased; 2: Increased |
| C. Serous Macular Detachment (SMD): |
| 1 | 5 (100%) | 5 (100%) | 4 (80%) | 1 (20%) |
| 0 | | | 1 (20%) | 4 (80%) |
| 2 | | | | |
| 1: Present; 0: Resolved; 2: Increased. |
| D. Ellipsoid Zone (EZ): |
| 1 | 12 (100%) | 9 (75%) | 4 (33.33%) | 2 (16.66%) |
| 2 | | 3 (25%%) | 8 (66.6%) | 10 (83.33%) |
| 1: Disrupted; 2: Regenerated |
The IOP spike was observed in 5% of cases post IVB. The elevation in IOP was mild and did not require any specific treatment.
Post-injection mild anterior chamber reaction was observed in 5 eyes (15.62%), but it resolved within 7-10 days with topical corticosteroids. At six months follow-up, no other serious ocular or systemic adverse effects to the injection were reported.
The cases that did not show complete resolution of macular oedema on OCT after first injection were subjected to repeat IVB. Of the total 32 eyes, 16 eyes (50%) received second injection of IVB after reviewing OCT at 1st month follow-up and 9 eyes (28.12%) received third injection IVB after reviewing OCT at 3rd month follow-up. At six months follow-up, OCT of 5 (15.62%) eyes did not show total resolution of macular oedema. Two patients (6.25%) did not require a second injection as macular edema in them resolved with a single injection. Hence, 1 eye was subjected to pars plana vitrectomy and remaining 4 eyes received injection intravitreal Triamcinolone acetate [Table/Fig-7,8,9 and 10].
The baseline SD-OCT shows disruption of foveal contour, intraretinal cystoid spaces, HRF and distortion of EZ with Central Macular Thickness (CMT) on ETDRS grid.
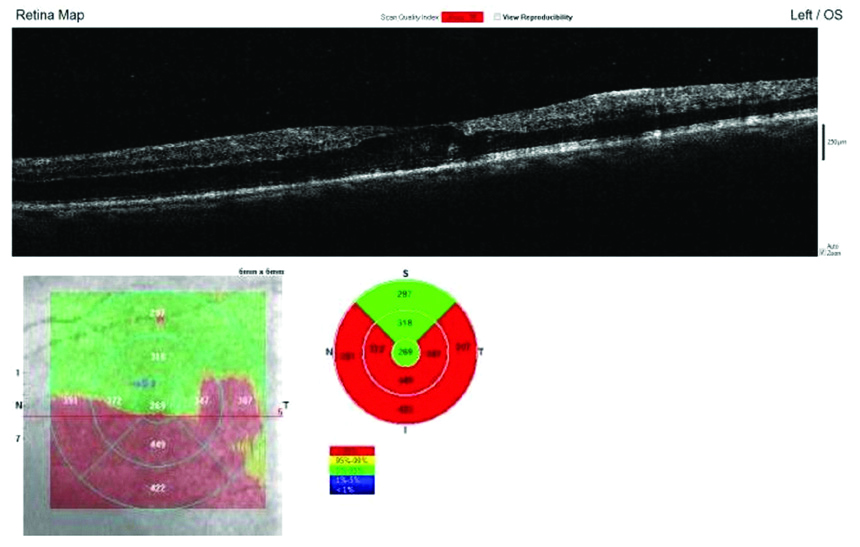
At 1 month follow-up, SD-OCT shows restoration of foveal contour, decrease in intraretinal cystoid spaces and HRF, distortion of EZ with decrease in CMT on ETDRS grid.
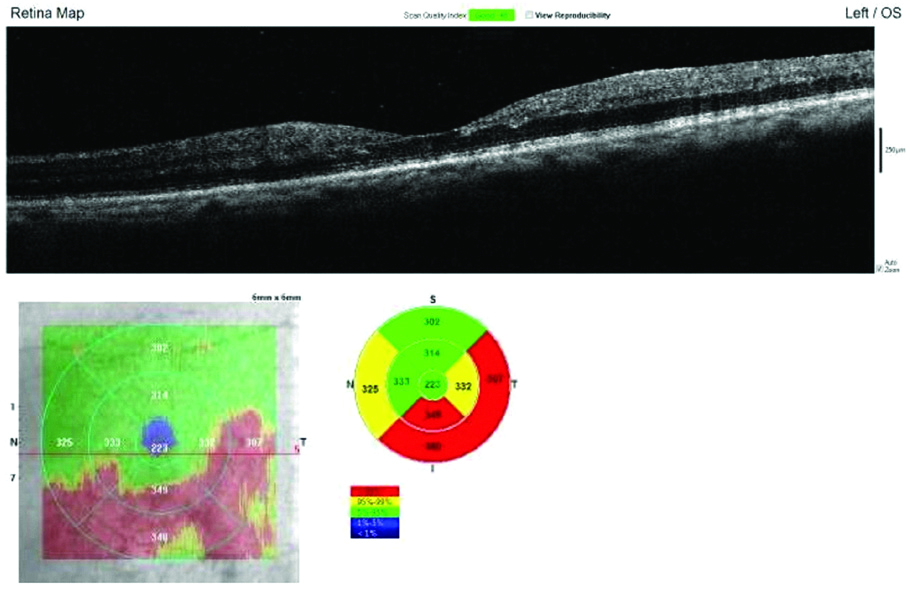
At 3 months follow-up, SD-OCT shows normal foveal contour, total resolution in intraretinal cystoid spaces and HRF, regenerating EZ, improvement of CMT on ETDRS grid.
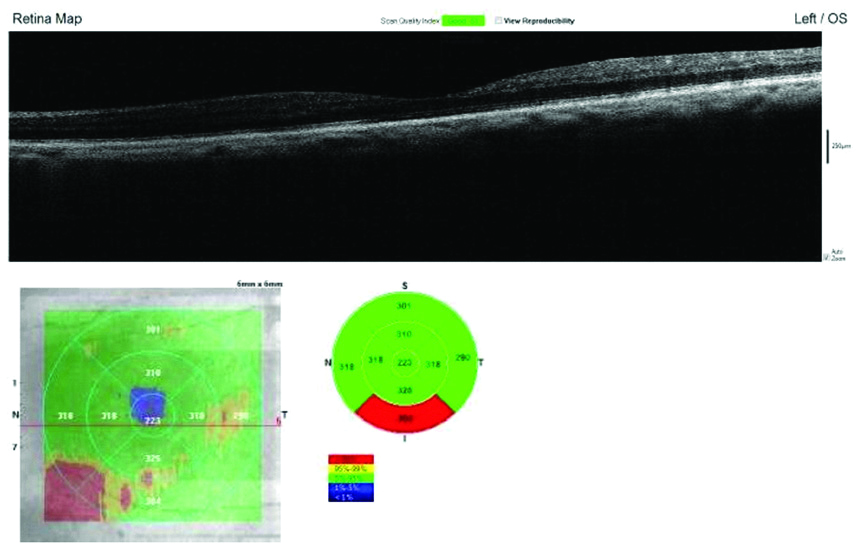
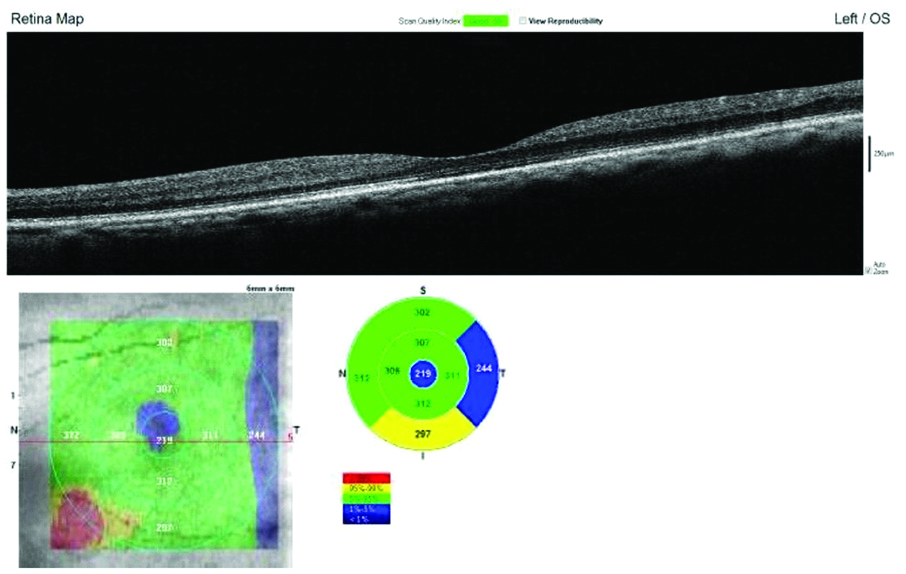
At 6 months follow-up SD-OCT shows normal foveal contour, total resolution in intraretinal cystoid spaces and HRF, regeneration of EZ with normal CMT on ETDRS grid compared to baseline.
Discussion
The result of this study demonstrated that IVB is efficacious as a primary treatment of macular oedema as 84.37% of eyes showed improvement in macular oedema as seen on SD-OCT and improvement in vision as well. According to pro-renata regimen, 16 eyes (50%) were given a second injection of IVB after 1st month follow-up and 9 eyes (28.12%) were given a third injection IVB after the 3rd month follow-up.
Also, the present study assessed other OCT morphological changes like cystoid spaces, SMD, HRF, EZ which influenced the final functional outcomes.
The BOLT study [7] found that the central macular thickness reduced from 507±145 μm at baseline to 378±134 μm (p<0.001) at 12 months post-injection IVB, whereas it reduced to a lesser extent in the laser group, from 481±121 μm to 413±135 μm (p=0.02) and the mean ETDRS BCVA at 12 months was 61.3±10.4 in the IVB group and 50.0±16.6 in the laser arm (p=0.006).
The VIBRANT study [8] was a double masked, multicenter trial, conducted to study intravitreal aflibercept for macular oedema following branch RVO and compared the drug to macular laser. The study concluded anti-VEGFs to be superior to laser. Hence, anti-VEGFs acquired increasing intraocular use and this caused a paradigm shift in the treatment of macular oedema. It is now being used intravitreally for DME, Retinal Vein Occlusion (RVO), Choroidal Neovascular Membrane (CNVM) and for stage 3+retinopathy of prematurity.
The MARVEL study [9] was a randomised, double-masked, controlled study of the efficacy and safety of IVB versus ranibizumab in the treatment of macular oedema due to branch RVO, which concluded both the drugs to be equally effective.
A number of head to head trials namely CATT trial, IVAN trial, MANTA, LUCAS and GEFAL were conducted worldwide [10-14] on a wide range of patients to asses the efficacy of FDA approved ranibizumab, over bevacizumab. The CATT study measured the mean change in visual acuity over a period of two years and concluded bevacizumab to be equally effective as ranibizumab. GEFAL and MANTA trial concluded that bevacizumab is no inferior to ranibizumab in efficacy and safety. However, the added advantage of bevacizumab is its cost effectiveness, which has given it an upper hand especially in a developing country like India.
In light of the above information, this retrospective study was conducted to review eyes with macular oedema receiving at least one IVB followed-up for a period of six months.
The results of this study indicate that bevacizumab increases visual acuity, reduces macular thickness and improves morphology irrespective of the type of macular oedema (focal or diffuse) and the retinal pathology causing it. Over the time, the number of patients requiring reinjection reduced from 50% to 28.12%, which indicates bevacizumab might have a beneficial cumulative effect, but further studies are necessary to prove this.
Arevalo JF et al., conducted a similar study on primary IVB for treatment of diffuse DME and found that mean central macular thickness at baseline by OCT was 387.0±182.8 μm and decreased to a mean of 287.9±102.4 at end of one month which was statistically significant; reduced to 282.8±115.6 μm at three months and 275.7±108.8 μm at six months, which was not statistically significant from the 1st month [15]. Similarly in this study, CFT was 445.28±155.87 μm at baseline that decreased to a mean of 334.44±100.42 μm, which was statistically significant; reduced to 332.84±101.20 μm at three months and 330.75±103.17 μm at six months, which was not statistically significant from the 1st month.
In the above study final BCVA analysis demonstrated that 32 (41.1%) eyes remained stable, 43 (55.1%) improved ≥2 ETDRS lines of BCVA, and 3 (3.8%) decreased ≥2 ETDRS lines of BCVA [15]. Similar changes were also noted in this study. Tehmina J et al., Lam DS et al. and Hou J et al., also conducted similar studies to the present study [16-18].
Jain P et al., in their study found endophthalmitis in 0.08% cases and IOP rise in 0.9% cases [6]. Similarly in this study, IOP spikes occurred in 5% of cases post-injection, which came down to normal without any treatment. Sermsiri S et al., in their study found that non-fatal myocardial infarction or IHD occurred only 0.33% of cases in the IVB group [2]. Campbell RJ et al., found that neither ranibizumab nor bevacizumab was associated with significant risks of ischemic stroke, acute myocardial infarction, congestive heart failure, or venous thromboembolism [19]. This study showed similar results as, there were no serious ocular and systemic adverse effect with the drug.
Limitation(s)
This study was short termed, non-randomised and retrospective. Due to this, the study was unable to estimate the long-term efficacy and safety of the drug. As the study is non-randomised with no control group, it cannot infer if some improvement in macular oedema was due to improvement in systemic health of the patients.
Conclusion(s)
IVB in present study was found to be effective for treatment of macular oedema due to retinal pathologies because of DME, RVO, CNVM and hypertensive retinopathy with macular star. Certain pathology, due to early detection and diagnosis, responded to a single injection, whereas others required two to three injections. OCT was found to be very effective tool in qualitative and quantitative assessment of macular oedemas along with microstructural features.