Bartonella Endocarditis: A Case Series from Chennai, India
Rayvathy Balasubramanian1, Pierre Edouard Fournier2, Panneer Selvam Ganesan3, Thangam Menon4
1 Assistant Professor, Department of Microbiology, Dr. ALM PG Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai, Tamil Nadu, India.
2 Professor, Department of Microbiology, UMR VITROME, Aix-Marseille University, Institut Hospitalo-universitaire Méditerranée Infection, Marseille, Provence-Alpes-Côte d’Azur, France.
3 Senior Resident, Department of Cardiology, Madras Medical College and Rajiv Gandhi General Hospital, Chennai, Tamil Nadu, India.
4 Professor and UGC BSR Faculty, Department of Microbiology, Dr. ALM PG Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Thangam Menon, Department of Microbiology, Dr. ALM PG IBMS, University of Madras, Taramani, Chennai, Tamil Nadu, India.
E-mail: thangam56@gmail.com
Bartonella spp. are Gram-negative haemotropic bacteria transmitted by arthropod vectors. They are considered as important aetiologic agents of Blood Culture Negative Endocarditis (BCNE). The diagnosis of Bartonella endocarditis is often challenging because of its non-specific clinical presentation and difficulty in isolating the microorganism in culture using standard microbiological techniques as it is an intracellular bacterium. A combination of serological and molecular methods will aid in a comprehensive diagnosis of this condition. This study reports a series of four Bartonella endocarditis cases diagnosed in Chennai, Tamil Nadu, India. All of them were known Rheumatic Heart Disease (RHD) patients. The common valves affected in the patients were mitral and aortic valves. Three out of four cases showed presence of valvular vegetation in echocardiogram. One patient had a history of close contact with cats. Specific real time Polymerase Chain Reaction (PCR) targeting 16S-23S rRNA spacer region of Bartonella spp. was positive in three cases. Serology using Indirect Immunofluorescence Assay (IFA) was positive in one case. Western blot test was performed on serum samples of all four cases and Bartonella henselae was identified as the aetiological agent in one case. Extra cardiac complications were observed in three cases. Antibiotic treatment was successful in three out of four cases. One patient died due to acute renal failure.
Gram-negative bacilli, Infective endocarditis, Real-time polymerase chain reaction
Introduction
Blood Culture Negative Endocarditis (BCNE) is a serious condition that accounts for up to 76% of Infective Endocarditis (IE) cases in developing countries [1]. The genus Bartonella includes small, fastidious and facultative intracellular Gram-negative bacilli transmitted to humans by arthropod vectors. Bartonella spp. cause diseases in both immunocompromised and immunocompetent individuals [2], and are important BCNE agents, with a prevalence ranging from 0.2% to 12.7% of all cases of endocarditis [3]. Serology using indirect IFA is the common method used in diagnosis but cannot discriminate among Bartonella spp. due to cross reactions. Western blot test may help for identifying the causative species. Molecular diagnosis using specific real-time PCR assays allows rapid detection and identification of Bartonella spp. in blood or valve samples [4]. The present study reported retrospectively the clinical presentation and associated complications of Bartonella endocarditis cases diagnosed by serological and molecular methods in BCNE patients in Chennai, Tamil Nadu, India.
Case Series
Patients with endocarditis fulfilling the modified Duke’s criteria [5] but with negative blood culture, and admitted to the Department of Cardiology, Rajiv Gandhi Government General hospital, Chennai, Tamil Nadu, India between November 2016 and May 2019 were included in this study. Written consent was obtained from all patients. This study was approved by the Institutional human ethics committee (IHEC NO: UM/IHEC/F.RM/2019-X). Blood samples were collected in triplicates from each patient and blood culture was performed using standard methods [6]. Whole blood and serum were collected from all BCNE patients. DNA was extracted from whole blood using the QIAamp DNA Blood mini kit (Qiagen, GmBh, Germany). DNA and serum samples were stored at -20°C until further analysis. For real time Polymerase Chain Reaction (PCR) targeting the 16S-23S rRNA spacer region, specific primers and Taqman probe were used [Table/Fig-1] [7]. Serology by indirect IFA was done on serum and species identification was obtained by western blot coupled to cross-adsorption as previously described [8].
Primers and probe used to detect Bartonella spp. by real-time PCR [7].
| Primer and probe | Sequence 5’-3’ | Molecular target | Reference |
|---|
| Barto ITS3 FBarto ITS3 RBarto ITS3 P | 5’-GATGCCGGGGAAGGTTTTC-3’5’-GCCTGGGAGGACTTGAACCT-3’5’-FAM-GCGCGCGCTTGATAAGCGTG-TAMRA-3’ | 16S-23S rRNA spacer | Safont M et al., [7] |
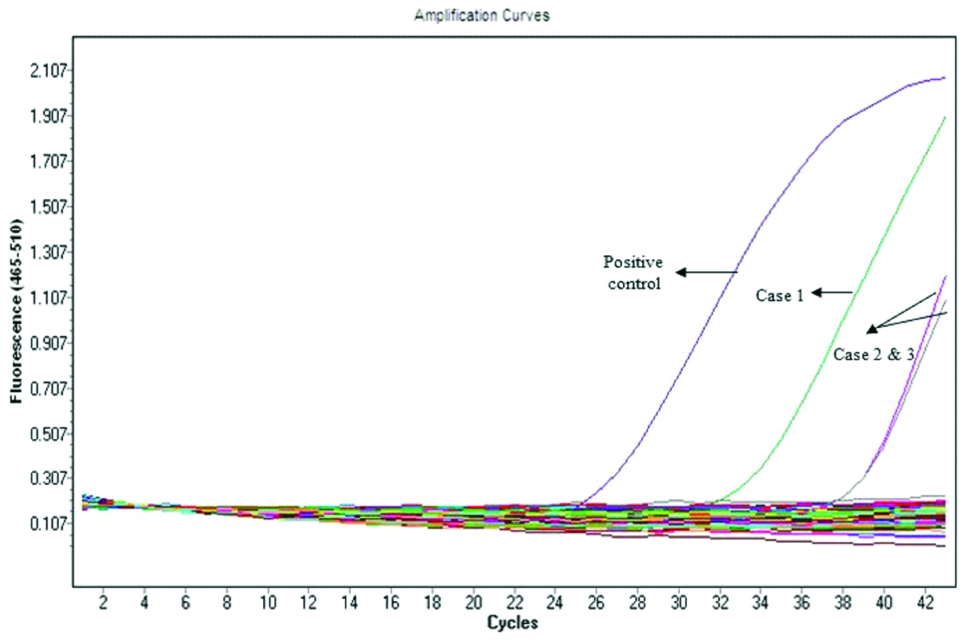
During the study period, a total of 85 patients with IE were admitted of these, 76 had negative blood cultures. DNA and serum obtained from these 76 patients were subjected to real time PCR (Light cycler 480, Roche life sciences) and IFA to detect evidence of Bartonella infection. Three cases of Bartonella endocarditis were detected using real time PCR [Table/Fig-2] and one case was detected by IFA. All these four cases were subjected to western blot and IFA positive sample yielded positive result and was speciated as B. henselae. The serum samples of three real time PCR positive cases were found to be negative in western blot analysis. The clinical and laboratory characteristics of Bartonella endocarditis cases are summarised in [Table/Fig-3].
Amplification curve of Bartonella real time PCR.
Clinical and laboratory data of patients with Bartonella endocarditis*.
| Patient | Age/Sex | Valve involved | Underlying cardiac condition/RF | Extracardiac disease | Contact with cats | Echocardiographic data | Vegetation | Bartonella serology | Bartonella Real time PCR | Anemia | ESR | Antibiotic treatment and outcome |
|---|
| I | 35/M | Mitraland aortic | RHD/P | Cellulitis in right leg. | Yes | AML, PML thickened, MS-Mild; MR- severe; AR- severe; PHT- moderate | 15 × 8 mm mobile vegetation attached to mitral chordae and 10 mm vegetation attached to aortic valve. | IFA and Wb-Negative | Positive;Ct 33.61 | Yes (Hb: 6 g/dL) | ND | Inj.Ceftriaxone 1 g IV BD × 4 weeks and Inj. Gentamicin 60 mg IV BD × 2 weeksPatient well and discharged |
| II | 16/M | Mitraland aortic | RHD/P | Nil | No | AML, PML thickened, MR- severe, AR- severe, TR- mild, mild PHT | No vegetation | IFA and Wb-Negative | Positive;Ct 38.93 | Yes (Hb: 6.4 g/dL) | Raised(103 mm/h) | Inj.Ceftriaxone 1 g IV BD × 4 weeks.Patient well and discharged |
| III | 33/M | Mitral | RHD/P | Left hemiparesis and Upper motor neuron facial palsy due to right MCA infarct | No | AML, PML thickened, PML mobility restricted, AML prolapsed and perforation +, MR, AR- severe, RCC-prolapse, mild PHT. | 3 × 5mm vegetation in RCC | IFA and Wb-Negative | Positive;Ct 38.99 | Yes (Hb: 8.7g/dL) | Raised (32 mm/h) | Inj.Cefotaxime 2 g IV three times a day × 6 weeks Inj.Gentamicin 60 mg IV BD × 4 weeks.Discharged in stable condition. |
| IV | 65/F | Mitral | RHD/N | AKI(Urea-114 mg/dL, creatinine-2,5 mg/dL) | No | AML, PML thickened, PML mobility restricted, MR-severe, TR- mild, moderate PHT | Freely mobile hyperechogenic mass of size 10 × 14 mm attached to atrial side of AML | IFA screening -PositiveWb-Positive B.henselae | Negative | Yes (Hb: 7.2g/dL) | Raised (40 mm/h) | Inj.Ceftriaxone 1 g IV BD x 1week, Inj. Linezolid 2 × 600 mg/day IV × 2 daysDeath due to acute kidney failure on 8th day of admission |
*RHD: Rheumatic heart disease; RF: Rheumatoid factor; MCA: Middle cerebral artery; AKI: Acute kidney injury; AML: Anterior mitral leaflet; PML: Posterior mitral leaflet; MS: Mitral stenosis; MR: Mitral regurgitation; AR: Aortic regurgitation; TR, Tricuspid regurgitation; PHT: Pulmonary hypertension; RCC: Right coronary cusp; IFA: Indirect immunofluorescence assay; Wb: Western blot; ESR: Erythrocyte sedimentation rate; P: positive; N: Negative; ND: Not done; Ct: Cycle threshold
All four patients were diagnosed as having definite endocarditis according to the modified Duke criteria. Three of the four patients were male and young, and their age was 16, 33 and 35 years respectively. The fourth patient was a 65-year-old female. All four patients had a history of RHD with valvular lesions, including three who had a positive rheumatoid factor. The mitral valve was affected in all patients, including two in whom the aortic valve was also affected. Valvular vegetations were present in three of the four cases. Three out of four patients were successfully treated with antibiotics and discharged. They were found to be normal in subsequent follow-up visits. One patient died because of acute renal shut down.
Discussion
Although many predisposing factors of Bartonella infections such as vectors, reservoirs, susceptible populations and suitable geographic conditions, are present in India, very few cases of Bartonella endocarditis have been reported to date [9,10]. The disease is likely underdiagnosed owing to the lack of awareness among clinicians who fail to consider it as a differential diagnosis in BCNE as well as to the non-availability of appropriate diagnostic methods in various clinical settings [4]. This study, here in report four patients diagnosed with Bartonella endocarditis using either IFA and western blot or specific real time PCR to a series of 76 patients with BCNE. Three were diagnosed by RT-PCR alone and one was diagnosed by IFA screening and western blot. Out of the four cases, three were male. Their mean age was 37.2±20.4 years. Combination of serology and molecular methods is useful in microbiological diagnosis of BCNE due to Bartonellosis in previous similar reports [11,12].
RHD was the major predisposing factor for IE in all four patients and a majority of these patients were young. Other studies in India have also reported that RHD remains highly prevalent in IE patients and that most IE cases occur in patients younger than 40-year-old [13,14]. Previous studies in African countries showed that endocarditis is a disease of young age with RHD as the common underlying disease [15]. A study done in Mediterranean region also reported that in southern countries, RHD remains a major risk factor of endocarditis and the patients were younger with high prevalence of culture negative endocarditis with zoonotic and arthropod-borne agents as frequent causative agents [3].
In this series, the mitral and aortic valves were the only involved valves, with vegetations being detected in 75% of cases. This was in accordance with the previous reports by Noopetch P et al., who reported a case of Bartonella henselae endocarditis with dissemination along with a brief review of seven Bartonella endocarditis cases in South East Asia. Aortic valve was the commonest affected valve and vegetations were present in majority of the cases [16].
Bartonella endocarditis presents clinically as subacute bacterial endocarditis with non-specific symptoms such as fever, fatigue and weight loss [17]. In this case series, three of the patients had fever as predominant presenting symptom. All patients suffered from anemia. In another study, Patel S et al., reported a case of Bartonella quintana endocarditis which presented with symptoms like productive cough, weight loss and intermittent abdominal pain. There was delay in diagnosis as it was originally diagnosed as non-infectious pathology leading to late initiation of antibiotic therapy [12].
Erythrocyte Sedimentation Rate (ESR) was elevated in three of the patients. Similar observation was made by Rodino KG et al., in a case report on Bartonella henselae endocarditis in a patient with previous history of RHD in whom the diagnosis was made by serology [18].
Patient I had an associated cellulitis in his right leg, which is a very rare complication of disseminated bartonellosis. A similar report by Fozard J et al., described B. henselae as causative agent of a periorbital cellulitis in a 12-year-old girl who had close contacts with a kitten [19].
Patient II was co-infected by Bartonella and Enterococcus faecalis. Co-infections in IE are rare but have been reported for zoonotic pathogens. Co-infections of Bartonella (with Pasteurella multocida and S. aureus) have been reported previously [20,21]. Co-infections between Bartonella spp. and Enterococcus faecalis have also been suspected, but a recent study demonstrated that serological cross-reactions between both pathogens were identified by western blot and the Bartonella co-infections were ruled out by PCR [22]. However, in this patient, molecular detection using specific real-time PCR assays demonstrated the presence of both Enterococcus faecalis and Bartonella.
Patient III presented with endocarditis complicated with stroke probably embolic in nature. Cases of Bartonella endocarditis complicated with embolic episodes have previously been reported by Tasher D et al., [23].
Patient IV was a female with IE and acute kidney injury. The kidney injury may be attributed to the immune-complex glomerulonephritis which is a complication of Bartonella endocarditis as previously described by Bookman I et al., [24].
Conclusion(s)
The current case series highlights the importance of Bartonella spp. as causative agents of BCNE in India, and demonstrates the usefulness of screening such patients for these microorganisms using a multi-disciplinary approach. It also depicts various rare aspects of the clinical presentation of Bartonella endocarditis.
*RHD: Rheumatic heart disease; RF: Rheumatoid factor; MCA: Middle cerebral artery; AKI: Acute kidney injury; AML: Anterior mitral leaflet; PML: Posterior mitral leaflet; MS: Mitral stenosis; MR: Mitral regurgitation; AR: Aortic regurgitation; TR, Tricuspid regurgitation; PHT: Pulmonary hypertension; RCC: Right coronary cusp; IFA: Indirect immunofluorescence assay; Wb: Western blot; ESR: Erythrocyte sedimentation rate; P: positive; N: Negative; ND: Not done; Ct: Cycle threshold
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 30, 2020
Manual Googling: May 01, 2020
iThenticate Software: May 15, 2020 (10%)
[1]. Benslimani A, Fenollar F, Lepidi H, Raoult D, Bacterial zoonoses and infective endocarditis, AlgeriaEmerg Infect Dis 2005 11(2):216-24.10.3201/eid1102.04066815752438 [Google Scholar] [CrossRef] [PubMed]
[2]. Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D, Bartonella, a common cause of endocarditis: A report on 106 cases and reviewJ Clin Microbiol 2015 53(3):824-29.10.1128/JCM.02827-1425540398 [Google Scholar] [CrossRef] [PubMed]
[3]. Gouriet F, Chaudet H, Gautret P, Pellegrin L, de Santi VP, Savini H, Endocarditis in the Mediterranean BasinNew Microbes and New Infections 2018 26:S43-51.10.1016/j.nmni.2018.05.00430402243 [Google Scholar] [CrossRef] [PubMed]
[4]. Diddi K, Chaudhry R, Sharma N, Dhawan B, Strategy for identification & characterization of Bartonella henselae with conventional & molecular methodsIndian J Med Res 2013 137(2):380-87. [Google Scholar]
[5]. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Proposed modifications to the Duke Criteria for the diagnosis of infective endocarditisClin Infect Dis 2000 30(4):633-38.10.1086/31375310770721 [Google Scholar] [CrossRef] [PubMed]
[6]. Liesman RM, Pritt BS, Maleszewski JJ, Patel R, Laboratory diagnosis of infective endocarditisJ Clin Microbiol 2017 55(9):2599-608.10.1128/JCM.00635-1728659319 [Google Scholar] [CrossRef] [PubMed]
[7]. Safont M, Angelakis E, Richet H, Lepidi H, Fournier PE, Drancourt M, Bacterial lymphadenitis at a major referral hospital in France from 2008 to 2012J Clin Microbiol 2014 52:1161-67.10.1128/JCM.03491-1324478415 [Google Scholar] [CrossRef] [PubMed]
[8]. Fournier PE, Gouriet F, Casalta JP, Lepidi H, Chaudet H, Thuny F, Blood culture-negative endocarditis: Improving the diagnostic yield using new diagnostic toolsMedicine (Baltimore) 2017 96(47):e839210.1097/MD.000000000000839229381916 [Google Scholar] [CrossRef] [PubMed]
[9]. Chaudhry R, Kokkayil P, Ghosh A, Bahadur T, Kant K, Sagar T, Bartonella henselae infection in diverse clinical conditions in a tertiary care hospital in north IndiaIndian J Med Res 2018 147:189-94.10.4103/ijmr.IJMR_1932_1629806608 [Google Scholar] [CrossRef] [PubMed]
[10]. Balakrishnan N, Menon T, Fournier PE, Raoult D, Bartonella quintana and Coxiella burnetii as causes of endocarditis, IndiaEmerg Infect Dis 2008 14:1168-69.10.3201/eid1407.07137418598654 [Google Scholar] [CrossRef] [PubMed]
[11]. Lam JC, Fonseca K, Pabbaraju K, Meatherall BL, Case Report: Bartonella quintana endocarditis outside of the Europe-African gradient: Comprehensive review of cases within North AmericaAm J Trop Med Hyg 2019 100(5):1125-29.10.4269/ajtmh.18-092930793686 [Google Scholar] [CrossRef] [PubMed]
[12]. Patel S, Richert ME, White R, Lambing T, Saleeb P, A case of Bartonella quintana culture-negative endocarditisAm J Case Rep 2019 20:602-06.10.12659/AJCR.91521531026253 [Google Scholar] [CrossRef] [PubMed]
[13]. Padmaja K, Sudhaharan S, Vemu L, Satish OS, Chavali P, Neeraja M, Clinicomicrobiological spectrum of infective endocarditis-from a tertiary care centre in south IndiaIranian Journal of Microbiology 2017 9(5):257-63. [Google Scholar]
[14]. Senthilkumar S, Menon T, Subramanian G, Epidemiology of infective endocarditis in Chennai, South IndiaIndian J Med Sci 2010 64(4):187-91.10.4103/0019-5359.9735822718013 [Google Scholar] [CrossRef] [PubMed]
[15]. Nkomo VT, Epidemiology and prevention of valvular heart diseases and infective endocarditis in AfricaHeart 2007 93(12):1510-19.10.1136/hrt.2007.11881018003682 [Google Scholar] [CrossRef] [PubMed]
[16]. Noopetch P, Ponpinit T, Suankratay C, Bartonella henselae infective endocarditis with dissemination: A case report and literature review in Southeast AsiaID Cases 2018 13:e0044110.1016/j.idcr.2018.e0044130155407 [Google Scholar] [CrossRef] [PubMed]
[17]. Okaro U, Addisu A, Casanas B, Anderson B, Bartonella species, an emerging cause of blood-culture-negative endocarditisClin Microbiol Rev 2017 30(3):709-46.10.1128/CMR.00013-1728490579 [Google Scholar] [CrossRef] [PubMed]
[18]. Rodino KG, Stone E, Saleh OA, Theel ES, The Brief Case: Bartonella henselae endocarditis- A case of delayed diagnosisJ Clin Microbiol 2019 57:e0011410.1128/JCM.00114-1931451567 [Google Scholar] [CrossRef] [PubMed]
[19]. Fozard J, Pandya N, Pulikot A, Fish D, Malhotra R, Lake D, Periorbital cellulits- A mistaken diagnosis!BMJ Case Rep 2011 2011:bcr072011450810.1136/bcr.07.2011.450822688944 [Google Scholar] [CrossRef] [PubMed]
[20]. Robbins A, Fouilhé L, Job L, Andreoletti L, Bani-Sadr F, Concomitant Pasteurella multocida aortic endograft infection and Bartonella henselae endocarditisMedecine et Maladies Infectieuses 2015 45(10):424-26.10.1016/j.medmal.2015.09.00426472059 [Google Scholar] [CrossRef] [PubMed]
[21]. Barbier F, Fournier PE, Dauge MC, Gallien S, Raoult D, Andremont A, Bartonella quintana coinfection in Staphylococcus aureus endocarditis: Usefulness of screening in high-risk patients?Clin Infect Dis 2009 48(9):1332-33.10.1086/59782619344260 [Google Scholar] [CrossRef] [PubMed]
[22]. Arregle F, Gouriet F, Amphoux B, Edouard S, Chaudet H, Casalta J-P, Western Immunoblotting for the diagnosis of Enterococcus faecalis and Streptococcusgallolyticus infective endocarditisFrontiers in Cellular and Infection Microbiology 2019 9:31410.3389/fcimb.2019.0031431572688 [Google Scholar] [CrossRef] [PubMed]
[23]. Tasher D, Raucher-Sternfeld A, Tamir A, Giladi M, Somekh E, Bartonella quintana; An unrecognized cause of infective endocarditis in children in EthiopiaEmerg Infect Dis 2017 23(8):1246-52.10.3201/eid2308.16103728730981 [Google Scholar] [CrossRef] [PubMed]
[24]. Bookman I, Scholey JW, Jassal SV, Bartonella infection presenting with prolonged fever in a pediatric renal transplant recipientAm J Kidney Dis 2004 43:25-30. [Google Scholar]