Introduction
A novel Corona Virus (CoV) named “2019 novel corona virus” or “2019-nCoV” or “COVID-19” by the World Health Organisation (WHO) is responsible for the recent outbreak of pneumonia that started in early December, 2019 in Wuhan City, Hubei Province, China [1-3]. This outbreak is related to a large market of seafood and animal, and investigations are currently ongoing to determine the source of the infection. To date, thousands of human infections have been confirmed in China along with many exported cases across the globe [4].
A pneumonia swarm with unknown aetiology appeared in Wuhan City, Chinese province of Hubei in December 2019. Majority of the initial patients visited wet market for seafood, where they also sold other wildlife species. Initial virus isolation from human patients and molecular analysis showed that the pathogen was a new virus, first named 2019-nCoV, and eventually this disease is renamed by WHO as COVID-19 [5]. This new COVID-19 virus is now the 37th member of the Coronaviridae known to infect humans. The WHO declared this outbreak a Public Health Emergency of International Concern (PHEIC) on January 30 2020, with the deadly explosive increase of confirmed cases.
Evolution of Covid-19
Within weeks of the authorities recognising a novel outbreak, the virus was identified, isolated, and sequenced. The first genome was released on January 10, for SARS-CoV-2, the first diagnostic PCR assay on January 15, 2020. Sequencing of this corona virus has particularly moved at a fast pace, with currently more than 50 sequences shared publicly. This sequencing statistics has allowed us to learn important features of the virus.
COVID-19 viruses mainly cause infections of the respiratory and gastrointestinal tract. Prior to this, six types of human CoVs were identified. These include HCoV-NL63, HCoV-229E, HCoV-OC43, HCoVHKU1, SARS-CoV, and MERS-CoV [6]. In 2003, SARS posed a serious public health threat to the world, with significant negative impact on the economy in the affected areas. Subsequent studies have shown that the SARS-CoV originated from bats and interspecies transmission to humans occured via an intermediate host [7]. Another well-known animal derived corona virus is Middle East Respiratory Syndrome (MERS)-CoV, which has an even higher case-fatality rate but is rarely transmitted among humans.
The 2019-nCoV is similar to the SARS-like bat CoVs, while the SARS-CoVs are inherited from the SARS-like bat CoVs, suggesting that 2019-nCoV has close similarity to the SARS-like bat CoVs. In comparison, 2019-nCoV is much less related to the MERS-CoVs.
Common human CoVs can cause common colds and upper respiratory infections in immunocompetent individuals. Lower respiratory tract infections can occur in immunocompromised subjects and the elderly.
Other human CoVs causes epidemics with clinical severity like respiratory and extra-respiratory manifestations. The mortality rates for SARS-CoV, MERS-CoV, are up to 10% and 35%, respectively.
The SARS-CoV-2 or Corona virus is elliptical with a diameter of approximately 60-140 nm. The virus strains may be effectively inactivated by using lipid solvents which includes ether, ethanol, chlorine-containing disinfectant, peroxyacetic acid and chloroform [8-10].
Epidemiology/Pandemic Status
Data provided by the WHO Health Emergency Dashboard (April 16, 2020, 05:30 GMT+ 5:30) report 1,991,562 confirmed cases and 130,885 reported death cases worldwide since the beginning of the epidemic. European Region has 977,596 confirmed cases and 84,607 reported deaths. Region of the Americas has 673,361 confirmed cases and 27,336 reported death cases. Western Pacific Region has 124,204 confirmed cases and 4201 reported deaths. Eastern Mediterranean Region has 107,389 confirmed cases and 5395 reported deaths. South-East Asia Region has 20,287 confirmed cases and 936 reported deaths. African Region has 11,367 confirmed cases and 523 reported deaths. The total global COVID-19 deaths have exceeded 100,000. WHO has declared the risk assessment at global level is very high [11].
Transmission of CoV
Phylogenetic analysis reveals that SARS CoV-2 is closely related to a group of corona viruses such as SARS. Nevertheless, it remains unclear where the virus originated and how it was first transmitted to humans.
Corona viruses associated with SARS are widely prevalent in bats. We therefore, presume once again that wild animals could also be the source this time, although the exact animal host is not yet known. Since, the first COVID-19 case was directly linked with the exposure to the Wuhan seafood market, the animal-to-human transmission is presumed as the key mechanism. The successive cases were not related to the exposure mechanism. Hence, it was concluded that the transmission of CoV from human-to-human is possible.
Knowing the specific biophysical method of proteolytic enactment of this novel spike protein and the collaboration between this receptor binding domain with the host receptor ACE2 at physiological and endosomal pH would unveil how this COVID infection crossed species.
The transmission from human-to-human occurs through respiratory droplets generated by coughing and sneezing is believed. Analysis of data pertaining to the spread of SARS CoV-2 in China seems to signal that close contact between individuals is necessary for the transmission. Further studies are needed to understand the mechanisms of transmission.
Clinical Manifestation
Initially, the indications of COVID-19 were fever, cough, runny nose, sore throat and dyspnea. A few patients demonstrated atypical symptoms like diarrhoea and vomiting as reported Sun J et al., [7]. But, the clinical correlation could not be done since about 25.2% patients have atleast one or the other underlying medical conditions [12-17].
Huang C et al., delineated in his first report that patients experienced fever, dry cough, and dyspnea [1]. Chest modernised tomography (CT) scanning showed pneumonia with unusual findings in every cases.
Chinese authors classified the CDC report according to the severity and its seriousness. Non-pneumonic and mild pneumonic comes under mild category of the infection, dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, lung infiltrates >50% comes under severe category of the infection and respiratory failure, septic shock, and/or Multiple Organ Dysfunction (MOD) or Failure (MOF) comes under critical category of the infection [18].
Prevention
Prevention is the current strategy to limit the spread of cases. Isolation of patients for control of disease is necessary. For diagnosis and clinical care doctors and nurses should wear PPE (Personal Protective Equipments which includes face protection, goggles and mask or face shield, gloves, gown or coverall, head cover, rubber boots). For example, droplet, contact, airborne precautions ought to be adopted during sample (sputum, swab) collection by maintaining a strategic distance. WHO has declared some basic recommendations that must be followed as preventive measures [19].
In an observational study, it was discovered that people touch their own face on an average of 23 times per hour with their own hands, often with skin contact, followed by mouth, nose and eyes [20]. The most effective strategy is to wash their hands frequently and with handwash or sanitisers and not to touch face with hand or mouth to defend pathogens from possible transmission.
After an outbreak situation the viral load of corona viruses on inanimate surfaces is now established, disinfection seem plausible to reduce the viral load on surface, particularly the frequently touched surfaces in the immediate patient surrounding where it is possible to predict the maximum viral load. The WHO advices “to ensure appropriate environmental cleaning and disinfection procedures are to be followed consistently and correctly. Cleaning environmental surfaces thoroughly with water and detergent and the use of hospital-level disinfectants (including sodium hypochlorite) are effective and powerful procedures” [21].
Treatment
No specific antiviral therapy is prescribed for COVID-19 sufferers, and no vaccine is presently available. The treatment is symptomatic, and oxygen treatment is the major option for patients with severe infection [22].
Approximately, 15% of COVID-19 patients are suffering with severe illness who desperately need treatment. So, rather than coming up with new compounds that may additionally take years to develop, researchers and public health groups are seeking out repurpose tablets already authorised for different diseases [23]. They are also seeking out unapproved drug which could perform well in preclinical studies with the other two deadly viruses which give rise to SARS and MERS.
WHO has announced a large global trial, called SOLIDARITY, to find out if any repurposed drug or combination drug can be used in treatment for the infection of dangerous respiratory disease. It’s an unprecedented effort to collect robust scientific data rapidly during a pandemic.
Drugs (hydroxychloroquine/lopinavir/ramdesivir) that may slow or kill the SARS-CoV-2 can save the lives of basically sick patients and be administered prophylactically, to ensure medical or clinical workers and others from high danger of contamination. Experts have suggested several different existing compounds for testing but WHO focuses on what it assumes are the four most effective treatments: an antiviral compound called Remdesivir; the malarial drugs Chloroquine and Hydroxychloroquine; a mixture of two HIV drugs, Lopinavir and Ritonavir; and that combination with addition to interferon-beta, an immune booster that can help cripple infections.
Remdesivir
The SARS-CoV-2 is giving this compound a second chance to shine. Remdesivir was first developed by Gilead to control Ebola and associated viruses. Remdesivir closes viral replication by inhibiting the RNA-dependent polymerase enzyme.
Surprisingly, scientists from the Democratic Republic of Congo tested Remdesivir along with the other two drugs during Ebola outbreak period and found they have no potential effect at all [23]. In 2017, researchers from the University of North Carolina showed that the drug can inhibit corona viruses causing SARS and MERS [24].
A first COVID-19 patient was given Ramdesivir in the US when his condition was almost fatal; the next day as per the New England case report, Journal of Medicine (NEJM) the patient improved and survived [24].
Chloroquine and Hydroxychloroquine
President Donald Trump called Chloroquine and Hydroxychloroquine a “game changer” in a press conference. His statement has triggered the antimalarials to rush into demand. Since drugs have gained significant attention in many countries, according to the study report by a WHO working group that explored the potential of the drugs. The broad interest led to the need to examine the emerging evidence in order to report about its potential role. The available data are thin.
The drugs work by reducing endosomal acidity, compartments within cells are used to ingest foreign material and hence some viruses can co-opt to enter a cell. But the main entrance to SARS-CoV-2 is different, using its so called spike protein to bind to a receptor on the surface of human cell. Cell culture studies have suggested that Chloroquines have some activity against SARS-CoV-2, but the doses needed are usually high and could lead to serious toxicity.
In guidance published, the “US Society of Critical Care Medicine” said that “there is less evidence to release a advise on the rationale use of Chloroquine or Hydroxychloroquine in critically ill patients with COVID-19”. Especially, Hydroxychloroquine may do more harm than good as the medication has a variety of side effects and in rare case can be harmful to heart [25].
Ritonavir/Lopinavir
Brand name Kaletra, a combined form of Ritonavir and Lopinavir were affirmed in the US in 2000 to treat HIV. Abbott Laboratories was the only developer and producer of Lopinavir specifically for inhibiting HIV protease enzyme that cleaves a long protein chain into peptides when new viruses are gathered [26]. Since Lopinavir is rapidly broken down by our own protease in body, it is administered with low Ritonavir levels, another protease inhibitor which let Lopinavir persist longer. The combination may also inhibit the protease of other viruses, specifically corona viruses [26].
It has been shown to be effective in MERS infected marmosets and has also been tested in SARS and MERS patients, but the findings of the trials are unclear [26].
However, the first trial with COVID-19 patients was not successful. Doctors in Wuhan, China, gave two Lopinavir/ Ritonavir pills twice daily to 199 COVID-19 patients with standard care. But there was no significant difference between groups, they reported in NEJM on March 15, 2020. The authors cautioned that more than 1/5th of patients died and other patients were critically ill and that the medication may have been given too late to help [25]. Doctors and scientist have already warned it with symptomatic dose to be given to the ill patient when in combinations with other drugs or otherwise this drug is generally safe.
Discussion
Human corona viruses can remain infectious for up to nine days at room temperature or on inanimate surfaces. Therefore, contamination of regular contact surfaces in healthcare settings is a potential source of viral transmission.
Appropriate procedures for cleaning and disinfecting the surface must be followed consistently and correctly. Commonly used hospital-level disinfectants (such as Sodium Hypochlorite) are effective against virus [Table/Fig-1]. Surface sterilisation or disinfection with 0.1% sodium hypochlorite or 62-71% ethanol absolutely reduces contamination [Table/Fig-2] with corona virus on surfaces instantly in exposure [27].
Inactivation of corona virus by different concentration of sodium hypochlorite.
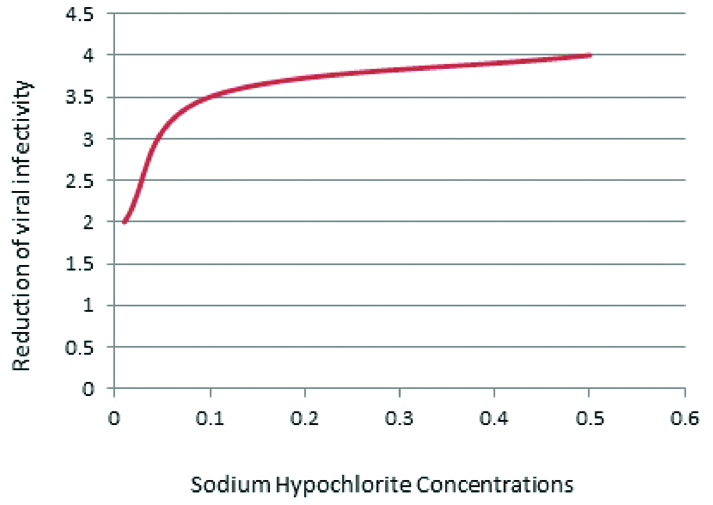
*Inactivation of corona viruses by different types of biocidal agents [27].
| Biocidal agent | Concentration | Virus | Strain/isolate | Volume/Material | Organic load | Exposure time | Reduction of viral infectivity (log10) |
|---|
| Ethanol | 70% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | >3.0 |
| Benzalkonium chloride | 0.04% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | <3.0 |
| Sodium hypochlorite | 0.5% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | >3.0 |
| Sodium hypochlorite | 0.1% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | >3.0 |
| Sodium hypochlorite | 0.01% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | <3.0 |
| Glutardialdehyde | 2% | HCoV | Strain 229E | 25 mL/ stainless steel | 5% serum | 1 min | >3.0 |
HCoV: Human corona virus; *depending on the volume of injected hydrogen peroxide
Long term complications are not yet known among survivors of SARS-CoV-2 infection with clinically relevant COVID-19 disease. Internationally, the death rates remain between 1% to 2%. First of its kind data suggest the death rate ranges from 1% to 2% with respect to the different countries. Most of the fatalities have happened in patients more than 50-year-old age group [28]. Kids have all the factors of being somewhat contaminated yet may fill in as a vector for additional transmission.
Globally, multiple studies are investigating the use of a broad-spectrum antiviral called Remdesivir.
The potential for these viruses to become a global pandemic appears to pose a serious risk to public health. As for COVID-19, on February 28, 2020, the WHO raised the danger to the CoV pandemic, to the high level. As the circumstance are changing quickly, the impact of the pandemic caused by the new CoV is still to develop.
World policy makers are trying to establish counter measures to prevent the possible catastrophic consequences. Health organisations are coordinating and organising information flows and issues and recommending guidelines to mitigate the impact of the threat. Simultaneously, researchers around the globe work tirelessly, and giving informations about the transmission systems, the clinical spectrum of SARS CoV-2, new diagnosis, prevention and therapeutic techniques are quickly developing as reported by Cascella M et al., [22].
There remain many uncertainties regarding the virus-host interaction and the evolution of the epidemic, with particular reference to the times when the epidemic reaches its top.
Conclusion(s)
Since the first outbreak of corona virus (COVID-19) in Wuhan, China, the disease has spread worldwide. Individuals at the peak of ages and those that are immunocompromised are at the highest risk. Countries are trying to establish counter measures to prevent the possible devastating effects. All the health organisations are issueing rules and guidelines to relieve the impact of the threat. Simultaneously, researchers, scientists around the globe working resolutely, and information about the transmission systems, the clinical range of COVID-19, new diagnosis, and prevention and therapeutic procedures are quickly developing by countries.
HCoV: Human corona virus; *depending on the volume of injected hydrogen peroxide
[1]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in WuhanLancet 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[2]. Zhou P, Yang X L, Wang XG, Hu B, Zhang L, Zhang W, Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat originBioRxiv 2020 579(7798):270-73.10.1101/2020.01.22.914952 [Google Scholar] [CrossRef]
[3]. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, A novel coronavirus from patients with pneumonia in China, 2019New England Journal of Medicine 2020 382(8):727-33.10.1056/NEJMoa200101731978945 [Google Scholar] [CrossRef] [PubMed]
[4]. China CDC (2020). Tracking.the.Epidemic.htp://weekly.chinacdc.cn/news/TrackingtheEpidemic.htm?from=timeline#Beijing%20Municipality% 20Update. [Accessed on 16th April] [Google Scholar]
[5]. Alexander EG, Susan CB, Ralph SB, Raoul J, Christian D, Anastasia AG, Severe acute respiratory syndrome-related coronavirus: The species and its viruses- a statement of the Coronavirus Study GroupBioRxiv 2020 [Google Scholar]
[6]. Tang Q, Song Y, Shi M, Cheng Y, Zhang W, Qin X, Inferring the hosts of coronavirus using dual statistical models based on nucleotide compositionScientific Reports 2015 :5.1715510.1038/srep1715526607834 [Google Scholar] [CrossRef] [PubMed]
[7]. Sun J, He WT, Wang L, Lai A, Ji X, Zhai X, COVID-19: Epidemiology, evolution, and cross-disciplinary perspectivesTrends in Mol Med 2020 26(5):483-95.10.1016/j.molmed.2020.02.00832359479 [Google Scholar] [CrossRef] [PubMed]
[8]. Drosten C, Günther S, Preiser W, Van der Werf S, Brodt HR, Becker S, Identification of a novel coronavirus in patients with severe acute respiratory syndromeN Engl J Med 2003 348(20):1967-76.10.1056/NEJMoa03074712690091 [Google Scholar] [CrossRef] [PubMed]
[9]. Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Isolation and characterization of viruses related to the SARS coronavirus from animals in southern ChinaScience 2003 302(5643):276-78.10.1126/science.108713912958366 [Google Scholar] [CrossRef] [PubMed]
[10]. Song HD, Tu CC, Zhang GW, Wang SY, Zheng K, Lei LC, Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and humanProc Natl Acad Sci U S A 2005 102(7):2430-35.10.1073/pnas.040960810215695582 [Google Scholar] [CrossRef] [PubMed]
[11]. WHO situation reports https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
[12]. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, ChinaJAMA 2020 323(11):1061-69.10.1001/jama.2020.158532031570 [Google Scholar] [CrossRef] [PubMed]
[13]. Yang Y, Lu Q, Liu M, Wang Y, Zhang A, Jalali N, Epidemiological and clinical features of the 2019 novel coronavirus outbreak in ChinaMedRxiv 2020 Feb 10.1101/2020.02.10.20021675 [Google Scholar] [CrossRef]
[14]. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive studyLancet 2020 395(10223):507-13.10.1016/S0140-6736(20)30211-7 [Google Scholar] [CrossRef]
[15]. Pongpirul WA, Pongpirul K, Ratnarathon AC, Prasithsirikul W, Journey of a Thai taxi driver and novel coronavirusNew England Journal of Medicine 2020 382(11):1067-68.10.1056/NEJMc200162132050060 [Google Scholar] [CrossRef] [PubMed]
[16]. Bastola A, Sah R, Rodriguez-Morales AJ, Lal BK, Jha R, Ojha HC, The first 2019 novel coronavirus case in NepalLancet Infect Dis 2020 20(3):279-80.10.1016/S1473-3099(20)30067-0 [Google Scholar] [CrossRef]
[17]. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, First case of 2019 novel coronavirus in the United StatesNew England Journal of Medicine 2020 382(10):929-36.10.1056/NEJMoa200119132004427 [Google Scholar] [CrossRef] [PubMed]
[18]. Wu Z, McGoogan JM, Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: Summary of a report of 72 314 cases from the chinese center for disease control and preventionJAMA 2020 Feb 24 10.1001/jama.2020.264832091533 [Google Scholar] [CrossRef] [PubMed]
[19]. WHO, Advice For Public, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. [Accessed on 16th April] [Google Scholar]
[20]. Kwok YL, Gralton J, McLaws ML, Face touching: A frequent habit that has implications for hand hygieneAm J Infect Control 2015 43(2):112-14.10.1016/j.ajic.2014.10.01525637115 [Google Scholar] [CrossRef] [PubMed]
[21]. WHO. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. WHO. 2020 Jan 25; Interim guidance. WHO/2019-nCoV/IPC/v2020.2. [Accessed on 12th April] [Google Scholar]
[22]. Cascella M, Rajnik M, Cuomo A, Dulebohn S, Napoli R, Features, Evaluation and Treatment Coronavirus (COVID-19). [Internet] 2020 March 20 [Google Scholar]
[23]. Kai Kupferschmidt, Jon Cohen, WHO launches global megatrial of the four most promising corona virus treatmentsScience 2020 Mar 22 10.1126/science.abb8497 [Google Scholar] [CrossRef]
[24]. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronavirusesSci Transl Med 2017 9(396)10.1126/scitranslmed.aal365328659436 [Google Scholar] [CrossRef] [PubMed]
[25]. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19New England Journal of Medicine 2020 :NEJMoa200128210.1056/NEJMoa200128232187464 [Google Scholar] [CrossRef] [PubMed]
[26]. WHO. “Solidarity” clinical trial for COVID-19 treatments. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. [Accessed on 16th April] [Google Scholar]
[27]. Kampf G, Todt D, Pfaender S, Steinmann E, Presistence of coronaviruses on inanimate surface and their inactivation with biocidal agentsJ Hosp Infect 2020 104(3):246-251.10.1016/j.jhin.2020.01.02232035997 [Google Scholar] [CrossRef] [PubMed]
[28]. Frost J, Corona virus curves and different outcomesStatistics by Jim 2020 Available at: https://statisticsbyjim.com/basics/coronavirus/ [Google Scholar]