Detection of rare targetable EGFR variant in Metastatic Non-small Cell Lung Carcinoma by next Generation Sequencing: A Case Report
Anurag Mehta1, Ullas Batra2, Mansi Sharma3, Sanjeev Sharma4, Shrinidhi Nathany5
1 Head and Director Laboratory Services and Research, Department of Molecular Diagnostics, Pathology, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
2 Senior Consultant, Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
3 Consultant, Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
4 Scientist C, Molecular Diagnostics, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
5 Senior Resident, Department of Molecular Diagnostics, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Shrinidhi Nathany, RGCI&RC, Sir Chotu Ram Marg, Sector 5, Rohini, New Delhi, India.
E-mail: drshrinidhinathany@gmail.com
Single gene assays for variants in Epidermal Growth Factor Receptor (EGFR) demonstrate actionable and sensitising mutations in majority of the cases. However, the emergence of next generation sequencing platforms has facilitated the detection of newer variants in the already known drivers of lung carcinomas, which may be clinically actionable. Mutations in exons 18 to 21 of EGFR are widely characterised in literature, and rare unusual mutations in these regions are constantly being demonstrated recently. This report describes a rare exon 18 insertion variant in EGFR gene in a 61-year-old patient of non-small cell lung carcinoma, which is potentially actionable, thus highlighting the need of next generation sequencing based platforms in this era of precision medicine.
E709 codon, Exon18, Epidermal growth factor receptor, Molecular testing
Case Report
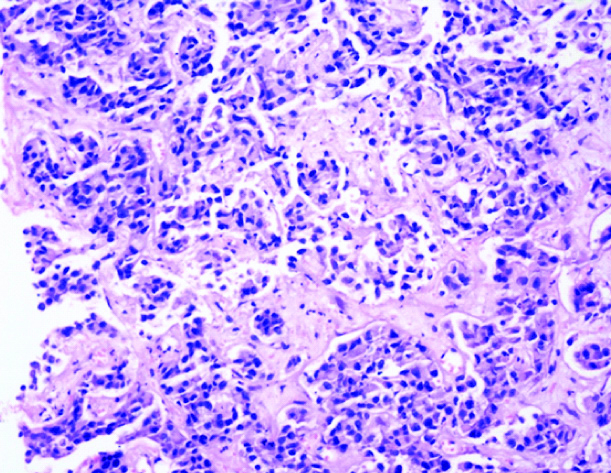
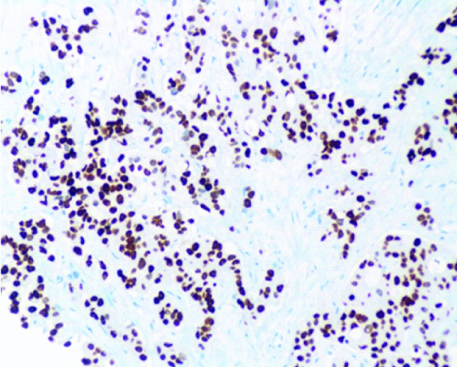
A 61-year-old male patient presented with the chief complaints of productive cough and occasional bouts of haemoptysis of 3-4 months’ duration. There was associated fatigue and dyspnoea on exertion. On physical examination, the patient had an obvious conjunctival pallor. However, there were no palpable lymph nodes, and the organ systems were unremarkable. There was no history of associated fever or any medically relevant history. Contrast Enhanced Computed Tomography (CECT) of thorax (evaluated elsewhere) revealed an approximately 5 cm × 5 cm mass in the lower lobe of right lung, along with tiny nodules in the upper lobe and a few small mediastinal lymph nodes. Routine hematologic and biochemical investigations were unremarkable. Bronchoscopy performed revealed infiltrates and bulges over right bronchus intermedius, suspicious of malignancy. A provisional diagnosis of a lung primary cancer. Histopathological and Immunohistochemical (IHC) examination of a core of the biopsy revealed a non-small cell lung carcinoma, favouring adenocarcinoma with a predominantly acinar pattern, composed of malignant cells, with moderate cytoplasm, irregular nuclei with dispersed chromatin, along with negative PDL1 (Programmed Death Ligand-1) staining. IHC was positive for Thyroid Transcription Factor 1 (TTF1), favouring the adenocarcinoma histology [Table/Fig-1a,b]. Molecular testing performed at this diagnostic time point was negative for sensitising mutations in EGFR {by real-time Polymerase Chain Reaction (PCR)}, using an FDA (Food and Drug Administration) approved kit by QiagenTM, USA), Anaplastic Lymphoma Kinase (ALK) (IHC) and ROS1(c-ros oncogene 1) rearrangements (by fluorescence in-situ hybridisation). Positron Emission Tomography (PET-CT) scan revealed a metabolically active right lung lesion with lymph node and bony involvement, mild right sided pleural effusion, pleural and bony (rib and ischium) deposits. The patient was started on Pemetrexed (500 mg/m2) and Cisplatin (75 mg/m2 in divided doses) based chemotherapy and 6 cycles of the same were administered.
Tissue biopsy of lung mass demonstrating acinar pattern of growth. H&E Section (X400).
Section showing diffuse nuclear expression of TTF1 confirming the diagnosis of Non-small Cell Carcinoma Lung- Adenocarcinoma (IHC, X400).
Post-six cycles PET CT re-evaluation revealed good partial response to treatment with residual metabolically active right lung lesion with bony involvements and right pleural deposits with pre-dominantly metabolically inactive mediastinal lymph nodal abnormalities. Magnetic Resonance Imaging (MRI) brain showed no metabolically active or enhancing parenchymal/meningeal lesion in brain. The patient’s performance status dipped at this follow-up.
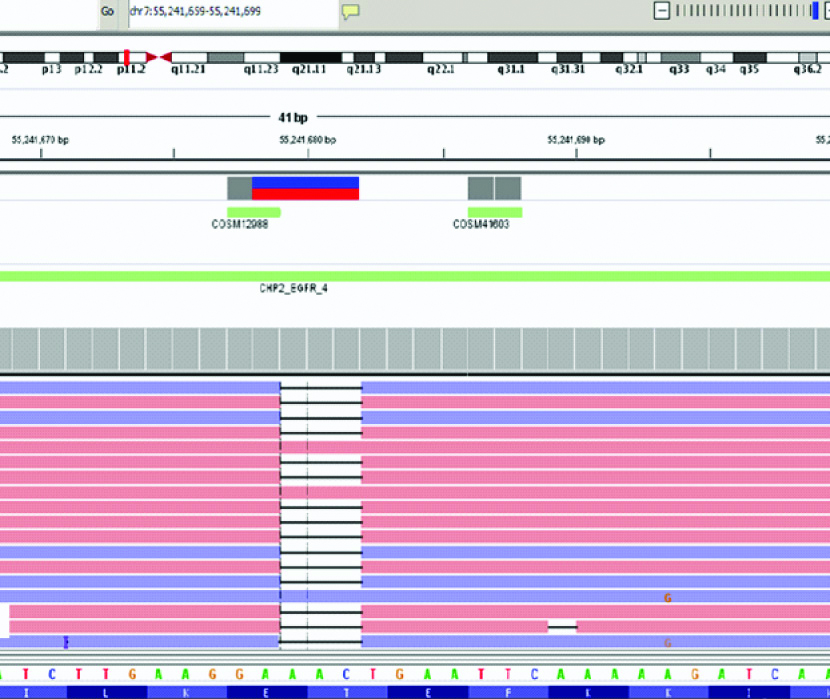
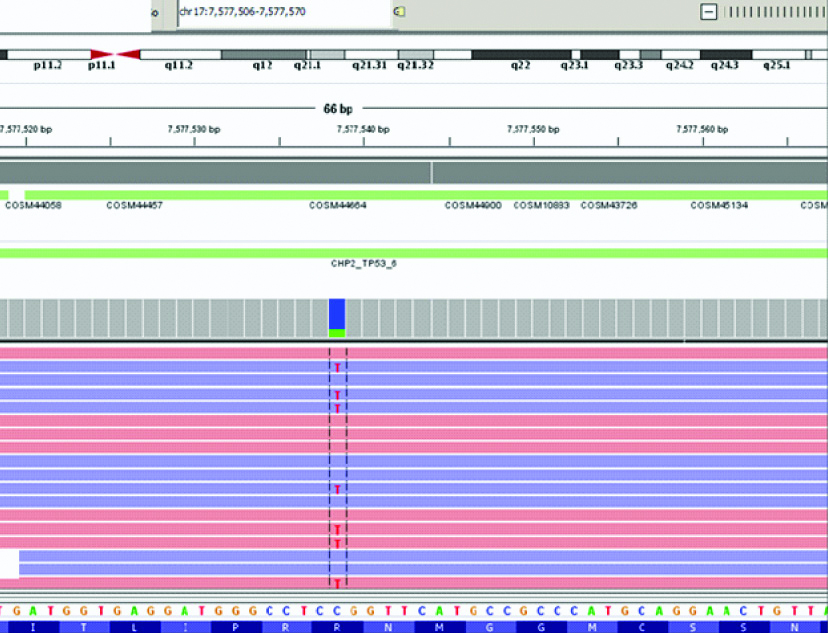
At this point, the original tumour was subjected to next generation sequencing using Oncomine Focus Assay Kit (ThermoFischer Scientific, Lifetechnologies Inc., USA) and the libraries were sequenced on Ion Torrent S5 platform. DNA based assay revealed a mutation in exon 18 of the EGFR gene which was p.(E709_T710delinsD):c.2127_2129delAAC [Table/Fig-2], and a mutation in TP53 gene: p.R248Q [Table/Fig-3] while the real-time PCR for EGFR was negative for any mutations. In view of the EGFR mutation detected, the patient was started on oral tyrosine kinase inhibitor therapy: Afatinib which was continued for seven months, during which the patient’s general condition was fair.
Integrative Genomics Viewer image depicting the mutation in the EGFR gene: a deletion was noted {p.(E709_T710delinsD)}.
Integrative Genomics Viewer image depicting the mutation in the TP53 gene: a missense mutation was noted.
However, subsequently, the patient presented with increasing cough, and a re-evaluation done using PET CT revealed increase in extent and metabolic activity of the right lung lesion causing complete distal collapse of lower lobe. Metabolically, active right pleural deposit was noted which was not seen earlier. Lymph nodes were unchanged. Left rib lesion showed increase in extent with persistent metabolic activity. Right ischial lesion showed significant increase in metabolic activity; new lesions were noted in the pleura on the right side, left rib, and right ischium bone, indicating progressive disease. The patient is now being treated with second line (Gemcitabine and Carboplatin) based on chemotherapy.
Discussion
Tier 1 mutations including EGFR, ALK, ROS1, NTRK and BRAF V600E form the first line testing as per the NCCN guidelines for lung malignancies. They are described in ~40% cases of NSCLC, with EGFR occurring in 25% cases of NSCLC [1,2]. The most common somatic mutations in the EGFR gene include inframe deletion of exon 19 reported in ~45% cases [3], and single nucleotide variants in exon 21; L858R in ~40% cases [3], which have shown dramatic response rates and better Progression Free Survival (PFS) when treated with oral EGFR TKIs (Gefitinib, Erlotinib). Rare EGFR mutations, such as G719X (~3%) and the L861Q (~2%) show higher rates of response and a prolonged PFS [4-6]. Other less common EGFR mutations have not been completely characterised, for example, the exon 18 deletion/insertion mutation delE709_T710insD described here. This mutation has been reported in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database [7] in 14 NSCLC cases as well as in My Cancer Genome (2 cases) [6]. In a report on rare EGFR mutations, Sousa AC et al., reported alterations in the E709 codon with one patient harbouring the same mutation and was not treated with first line TKI [7]. They reported an overall survival of 17 months in their case. On the second follow-up, the patient was in clinical stage IVB and he was administered Erlotinib, subsequent to which he showed a PFS of 10 months. However, unlike the present scenario, this case did not harbour any other concurrent mutations, which may explain the better PFS in their patient.
The overall reported frequency of this mutation is ~0.1% [8,9] in previous reports, and almost all of them have shown increased sensitivity and response to Erlotinib [5,9]. This patient, however, was treated with afatinib, and showed only partial response, eventually culminating in a progressive disease. The plausible explanation for the same can also be attributed to the co-existent pathogenic variant in the TP53 gene which may induce resistance, as has been well documented in literature. The TP53 mutation detected in the present patient has been reported pathogenic and confirmed somatic in the International Agency for Research on Cancer (IARC) TP53 database [10].
Conclusion(s)
This variant is very rare and is usually missed on single gene testing, which is widely done in NSCLC cases. This report highlights the importance of next generation sequencing based assays, in the detection of newer mutations which may be potentially actionable and can benefit the treatment and overall outcomes of a patients. However, the confirmation of the same requires in-vitro studies.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 09, 2020
Manual Googling: May 01, 2020
iThenticate Software: May 15, 2020 (13%)
[1]. Collisson EA, Campbell JD, Brooks AN, Berger AH, Lee W, Chmielecki J, Comprehensive molecular profiling of lung adenocarcinoma: The cancer genome atlas research networkNature 2014 511(7511):543-50.10.1038/nature1338525079552 [Google Scholar] [CrossRef] [PubMed]
[2]. Rossi S, D’Argento E, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Different EGFR gene mutations in Exon 18, 19 and 21 as prognostic and predictive markers in NSCLC: A single institution analysisMol Diagnosis Ther 2016 20(1):55-63.10.1007/s40291-015-0176-x26645830 [Google Scholar] [CrossRef] [PubMed]
[3]. Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC, Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in nonsmall cell lung cancerClin Cancer Res 2011 17(11):3812-21.10.1158/1078-0432.CCR-10-340821531810 [Google Scholar] [CrossRef] [PubMed]
[4]. Passaro A, Prelaj A, Bonanno L, Tiseo M, Tuzi A, Proto C, Activity of EGFR TKIs in Caucasian Patients With NSCLC Harbouring Potentially Sensitive Uncommon EGFR MutationsClin Lung Cancer 2019 20(2):186-94.10.1016/j.cllc.2018.11.00530563752 [Google Scholar] [CrossRef] [PubMed]
[5]. Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, COSMIC: The Catalogue Of Somatic Mutations In CancerNucleic Acids Res 2019 47(D1):D941-47.10.1093/nar/gky101530371878 [Google Scholar] [CrossRef] [PubMed]
[6]. Kusnoor SV, Koonce TY, Levy MA, Lovly CM, Naylor HM, Anderson IA, My cancer genome: evaluating an educational model to introduce patients and caregivers to precision medicine informationAMIA Jt Summits Transl Sci 2016 2016:112-21. [Google Scholar]
[7]. Sousa AC, Silveira C, Janeiro A, Malveiro S, Oliveira AR, Felizardo M, Detection of rare and novel EGFR mutations in NSCLC patients: Implications for treatment-decisionLung Cancer 2020 139:35-40.10.1016/j.lungcan.2019.10.03031715539 [Google Scholar] [CrossRef] [PubMed]
[8]. Ackerman A, Goldstein MA, Kobayashi S, Costa DB, EGFR delE709-T710insD: A rare but potentially EGFR inhibitor responsive mutation in nonsmall-cell lung cancerJ Thorac Oncol [Internet] 2012 7(10):e19-20.10.1097/JTO.0b013e3182635ab422982663 [Google Scholar] [CrossRef] [PubMed]
[9]. Gaughan EM, Costa DB, Genotype-driven therapies for nonsmall cell lung cancer: Focus on EGFR, KRAS and ALK gene abnormalitiesTher Adv Med Oncol 2011 3(3):113-25.10.1177/175883401039756921904575 [Google Scholar] [CrossRef] [PubMed]
[10]. Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, TP53 variations in human cancers: New lessons from the IARC TP53 Database and genomics dataHum Mutat 2016 5(1):7-20.10.1002/humu.2303527328919 [Google Scholar] [CrossRef] [PubMed]