Worldwide, the incidence and prevalence of nephrolithiasis ranges from 1-13%, with decreasing rates from United states (7-13%) to Europe (5-9%) and Asia (1-5%) [1]. Among the urologic diseases, nephrolithiasis is the most common in Asia [2]. Such differences in the rates among various regions are accounted by the difference in the genetics, age, climate, diet, race and metabolic diseases [3].
Urinary calculi are crystalline structures composed most commonly of calcium oxalate salts [4]. The course of management of urinary stones depends on a host of factors including location, size, composition, degree of obstruction, symptom severity, patient expectations, associated infections, other anatomical and medical issues and technical factors [5].
DECT has emerged as a viable option that can be helpful in determining the composition of urinary stones with precision under in-vivo conditions itself [8]. DECT is a simple, cost-effective and new technique which works on the principle of using two spectral X-ray beams (140kV and 80-100kV) to obtain two different images of the same tissue/region based on the absorption and attenuation index of the different material compositions. Based on this principle, DECT has been put to use in material differentiation (bone removal in angiography [9], bone marrow oedema [10], metal artefact reduction [11], material decomposition and iodine mapping (among lesions of lung, liver, adrenal and bowel pathologies) [12] and material separation (characterisation of urinary stones) [6,13,14]. DECT is able to differentiate the chemical nature of the stones based on their specific dual energy ratio, [12] and among the previous studies, Thomas C et al, Ascenti G et al., has been successful in characterisation of renal stones with DECT [13,14]. In another study, Li ZX, specifically used DECT to differentiate uric acid stones from non-uric acid stones in the patients with Gout and found significant results [6].
The characteristion of renal stones as shown in few of the previous studies is important as it may guide the therapy. The various types of urinary stones are managed differently based on the stone location, size, composition, and attenuation indices, and thus a pre-operative knowledge regarding this is warranted. Kambadakone AR et al., showed that uric acid stones (<400 HU) are treated by alkalinization of the urine that facilitates dissolution, cystine stones, non-uric acid stones (>1000 HU, >1 cm) are managed with ureteroscopy, or Percutaneous Nephrolithotomy (PCNL), and non-cystine non-uric acid stones (<1000 HU, <1 cm) can be best managed with Shock Wave Lithotripsy (SWL), or ureteroscopy [15].
Keeping in view the importance of urinary stone composition in determining the management and treatment course of patients, dual energy CT could be helpful in giving right direction to the treatment planning and reducing the load of unnecessary surgical interventions or in specifying the surgical intervention need. Hence, the present study was planned with an aim to determine the pure composition of renal stones with DECT.
Materials and Methods
A cross-sectional study was conducted in the Department of Radio-diagnosis in collaboration with Departments of Surgical Urology and Medicine, from November 2016 to May 2018. The study was approved by the Institutional Ethical Committee (ELMC/R_cell/EC/2017/16) and all expenses related to the use of DECT was borne by the hospital.
The sampling frame was bound by following inclusion and exclusion criteria:
Inclusion criteria
Patient diagnosed for renal calculi on ultrasound, Intravenous Pyelogram (IVP), X-ray Kiney Ureter and Bladder (KUB) and scheduled to undergo surgical extraction of stones, patient aged between 20-70 years, patient willing to give consent.
Exclusion criteria
Pregnancy, age <20 years, previously diagnosed cases of nephrolithiasis (on treatment), medically managed patients, patient not willing to give consent.
Sample Size
The proposed sensitivity of technique was 92.5 % [12]. Taking these values as reference, the minimum required sample size with desired precision of 12%, 95% power of study and 5% level of significance is 95 patients. To reduce margin of error, total sample size taken was 100.
Formula used is for testing sensitivity and specificity of single diagnostic test:
For sensitivity
n=(Zα/2×√Se×(1-Se)±Zβ×√(Se1*(1-Se1))2/difference2
where Se is sensitivity
Zα/2 is value of Z at two sided alpha error of 5% and Zβ is value of Z at power of 95%
Calculations:-
1) Sensitivity
H0:Se=92.5 versus Se≠92.5 (Se1)
With 95% confidence level and 95% power for detection of difference of 12% from a Se of 92.5%, sample size calculated is:-
N=((1.96*sqrt(.925*(1-.925))±(1.645*sqrt(.805*(1-.805))2/(.12*.12)
=94.73=95(approx.)
All the patients falling in sampling frame were invited to participate in the study. After obtaining an informed consent, demographic information, was noted. All the patients underwent ultrasonographic assessment for number and size of calculi. In case of multiple stones, the size of the largest stone was taken as representative.
Following this, DECT assessment was performed using Siemens “SOMATOM-force (384 slice)” machine. Both qualitative and quantitative analyses were performed for the purpose of evaluation of composition of the stones. After the patient was made to lie down on the scanner table, the area of interest was scanned with 80 kVp and 140kVp one after another diring the same phase of respiration. The graphical analysis was done by Siemens 3D Syngo imaging software solution and the exact stone type was determined from the graph as shown in [Table/Fig-1].
Non contrast CT scan showing stones in the renal pelvis identified on DECT as Uric acid stone.
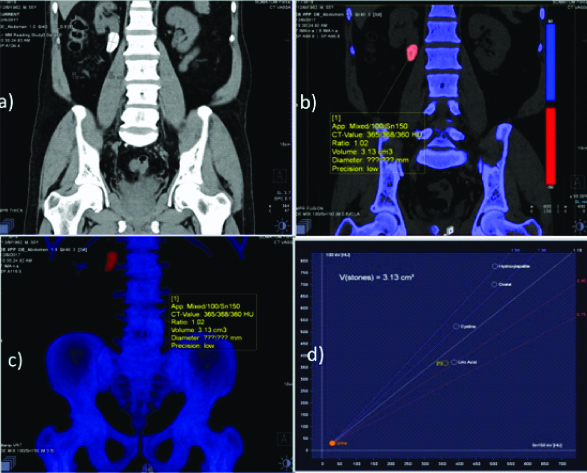
After the characterisation of the calculus via dual energy, patients were referred back for surgical extraction. Following surgical extraction of stones, they were subjected to laboratory analysis for determining the chemical composition. Composition determined by laboratory analysis was taken as the reference.
Findings of DECT and chemical composition were compared and appropriate management plans were discussed.
Statistical Analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean±SD. Diagnostic test was used to calculate sensitivity, specificity, PPV and NPV. Inter-rater kappa agreement was used to calculate strength of agreement between DECT analysis and chemical analysis. The data was entered in MS EXCEL spreadsheet and analysis was done using SPSS version 21.0.
Results
Age of patients ranged from 23 to 67 years. Maximum number of cases were in age group 31-40 years (40%). Mean age of patients was 41.15±10.08 years [Table/Fig-2].
Age Profile of Study Population (n=100).
| Age group (Years) | No. of cases |
|---|
| 21-30 | 14 |
| 31-40 | 40 |
| 41-50 | 28 |
| 51-60 | 12 |
| >60 | 6 |
Mean age±SD (range) 41.15±10.08 years
Majority of patients were males (64%). Sex ratio (M:F) was 1.78. Size of stones ranged from 1 mm to 40 mm. Majority of cases (55%) had stone size in 6-10 mm range and the mean stone size was 10.15±6.36 mm [Table/Fig-3].
Distribution according to stone size (n=100).
| Stone size (mm) | No. of cases |
|---|
| ≤5 | 13 |
| 6-10 | 55 |
| 11-20 | 26 |
| >20 | 6 |
Number of stones ranged from 1 to 9. Most of the cases (96%) had multiple stones. More than two third (68%) had three to five stones followed by >5 stones (18%), two stones (10%) and one stone (4%) respectively. Mean number of stones was 4.17±1.55.
Majority of stones i.e., 70% were blue in colour and 30% were red in colour. According to chemical analysis, maximum (32%) were identified as hydroxyapatite followed by cystine (30%), uric acid (28%) and oxalic acid (3%) respectively. There were 7% cases in whom chemical analysis detected mixed stones that included 3 cases with mixture of cystine±calcium oxalate and two each mixture of Calcium oxalate±hydroxyapatite and Calcium oxalate mixed respectively.
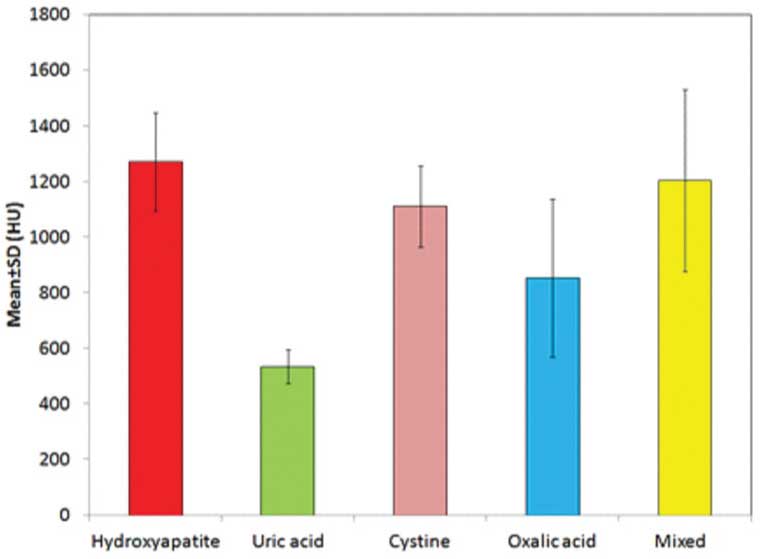
DECT pattern and perfusion quantitative analysis values were maximum for Hydroxyapatite (1271±178 HU) followed by mixed type (1203±327 HU), Cystine (1110±146 HU), Oxalic acid (853±284) and uric acid (534±62 HU) respectively. Statistically, there was a significant difference in DECT quantitative analysis for different chemical types of renal stones (p<0.001). [Table/Fig-4] However, as per the D/E ratio of the two scans, DECT assessed maximum number of stones as hydroxyapatite (36%) followed by cystine (34%) and uric acid (30%) respectively and it did not identify any stone as oxalic acid or mixed type.
DECT Quantitative analysis for different types of stones.
On chemical analysis hydroxyapatite was seen to be present in 34 stones (32 hyroxyapatite and 2 hydroxyapatite with Calcium oxalate). DECT identified hydroxypatite in 35 stones. As compared to chemical analysis, DECT had 32 true positive, 3 false positive, 2 false negative The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of DECT for hydroxyapatite stones and Hydroxyapatite with Calcium oxalate was 94.1%, 95.5%, 91.4%, 96.9% and 95%, respectively. The level of agreement between DECT and chemical analysis for hydroxyapatite stones was excellent (κ=0.889) as shown in [Table/Fig-5].
Agreement between Chemical and DECT Assessments: Hydroxyapatite (n=32) ± Hydroxyapatite with Calcium oxalate (n=2).
| DECT analysis | Chemical analysis | Total |
|---|
| Hydroxyapatite±Hydroxyapatite with calcium oxalate | Other types |
|---|
| Hydroxyapatite | 32 | 3 | 35 |
| Other types | 2 | 63 | 65 |
| 34 | 66 | 100 |
| Sensitivity | Specificity | PPV | NPV | Accuracy |
| 94.1 | 95.5 | 91.4 | 96.9 | 95.0 |
κ=0.889 (Excellent agreement)
On chemical analysis, 28 stones were identified as uric acid stones. On DECT, a total of 30 were identified as uric acid stones. As compared to chemical analysis, DECT had 28 true positive, 2 false positive, and no false negatives. Correspondingly, sensitivity, specificity, positive predictive value, negative predictive value and accuracy of DECT was 100%, 97.2%, 93.3%, 100% and 98% respectively for identification of uric acid stones. The level of agreement between DECT and chemical analysis for uric acid stones was excellent(κ=0.951) as shown in [Table/Fig-6].
Agreement between chemical and DECT assessments: uric acid (n=28).
| DECT analysis | Chemical analysis | Total |
|---|
| Uric acid | Other types |
|---|
| Uric acid | 28 | 2 | 30 |
| Other types | 0 | 70 | 70 |
| 28 | 72 | 100 |
| Sensitivity | Specificity | PPV | NPV | Accuracy |
| 100 | 97.2 | 93.3 | 100 | 98 |
κ=0.951 (excellent agreement)
On chemical analysis, a total of 30 stones were identified as cystine stones. On DECT 34 stones were identified as cystine stones. As compared to chemical analysis, DECT had 30 true positive, 4 false positive, and no false negatives. Thus, the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of DECT was 100%, 94.3%, 88.2%, 100% and 96%, respectively for identification of cystine stones. The level of agreement between DECT and chemical analysis for cystine stones was excellent (κ=0.908) as shown in [Table/Fig-7].
Agreement between chemical and DECT assessments: cystine (n=30).
| DECT analysis | Chemical analysis | Total |
|---|
| Cystine | Other types |
|---|
| Cystine | 30 | 4 | 34 |
| Other types | 0 | 66 | 66 |
| 30 | 70 | 100 |
| Sensitivity | Specificity | PPV | NPV | Accuracy |
| 100 | 94.3 | 88.2 | 100 | 96 |
κ=0.908 (excellent agreement)
DECT failed to detect any of the calcium oxalate (3 pure, 2 mixed and 2 in combination with hydroxyapatite) which could be termed to be the limitation of DECT.
Discussion
DECT is an expensive and not so commonly used imaging technique for the pre-characteristion of stones to guide the management. The current study results showed that DECT was useful for determining the chemical composition of renal stones.
In present study, on DECT, majority of stones (70%) were blue in colour with 30 (30%) stones red in colour. The DECT colour pattern varies as per the original colour of the stone. The colour of urinary calculi are diversified -the urinary apatite, consisting of hydroxyapatite has near white to pale brown colour, while uric acid stones are beige to yellowish-orange, pure cystine stones are yellow in colour and calcium oxalate stones are dark brown or sometimes black in colour which seems to look like blue [16]. Moreover, all the stones are not of pure type, and presence of mixed types might affect the actual colour of the stones. As such, owing to overlapping colours, the colour on DECT have little value in terms of determining the chemical composition of the stones.
The final chemical analysis of the stones were determined by spectroscopy. The index study showed hypdroxyapatite, uric acid, cystine stones/mixed and oxalate stones (in decreasing order). The results showed some variations as compared to other studies which may be due to the different diagnostic techniques, dietary habits and geographical locations of the study subjects. Previous studies reported maximum patients with calcium oxalate stones, [17-19] while few reported maximum patients with uric acid stones [20-21].
Statistically, there was a significant difference in DECT quantitative analysis for different chemical types of renal stones. Similar to present study, Li XH et al., also found significant differences in radiodensity of stones at 50 keV for uric acid (510.08±157.29 HU), struvite (1058.58±260.13), cystine (725.75±142.35 HU), calcium phosphate (2617.46±186.22 HU) and calcium oxalate (2617.46±186.22 HU) respectively [18]. Yadav B and Maharjan S in their study also showed significantly different mean HU values (at 80 kV) for different types of renal stones [19].
Barring the recognition of mixed stones and oxalate stones, the accuracy for prediction of other stones were excellent in the study; the reason being, the HU values of mixed stones and oxalate stones could not be segregated with perfection. In addition, in mixed stones, the HU values may vary depending upon the principal component present and thus it may show false negative results on DECT. One may consider it as the limitation of the technique itself since DECT characteristion of stones is based on the intensity of HU values and is not as accurate as spectroscopic detection of the stone components.
Similar to the present study, high accuracy of DECT in evaluation of different compositions of stones have been endorsed in different previous studies too. Kulkarni NM et al., in their study reported it to be 100% sensitive and accurate in detecting Uric Acid (UA) and non-UA stones [22]. In present study too, we found it to be 100% sensitive and 97.2% specific in detecting UA and non-UA stones. In another study, Krishna BC et al., reported DECT to have 100% sensitivity and specificity in differentiating uric acid stones from mixed and calcium oxide monohydrate stones [21]. Ilyas M et al., also found DECT to be 100% sensitive and specific for differentiating UA stones from the non-UA stones [23]. They also found that DECT had a sensitivity and specificity of 97.8% and 92.3%, respectively, in differentiating a calcium oxalate from non-calcium oxalate calculus. All these findings show that DECT is highly accurate in compositional analysis of urinary stones as observed in present study. It is to note that most of the previous studies categorised and identified the stones in two categories such as Uric acid and non-uric acid stones [21-23] or Calcium oxalate and non-calcium oxalate stones [23], thus increasing the prediction accuracy of the technique. This is mainly beneficial due to the management protocols for different stones- Uric acid stones being medically treated as compared to surgical treatment (PCNL, SWL) for other types of stones thereby reducing the burden of surgical management in many cases. In addition, the patients with Gout usually have Uric acid stones and the treatment thus can be specifically focused on the medications. In the present study, all the cases were managed surgically since the use of DECT was not diagnostic in the study but was done for the purpose of the study.
Limitation(s)
The non-identification of oxalate and mixed stones. This can be due to low prevalence of pure calcium oxalate stones (n=3), thus limiting the scope of present work to some extent.
Conclusion(s)
DECT can be used as a diagnostic tool for treatment planning of renal stones. It is a good tool to predict the morphological, chemical and anatomic features of renal stones. Larger studies are recommended for external validation.
Mean age±SD (range) 41.15±10.08 years
κ=0.889 (Excellent agreement)
κ=0.951 (excellent agreement)
κ=0.908 (excellent agreement)