Lymphangiomas are divided in three categories: capillary, cavernous and cystic. Cystic Lymphangioma (CL) are large, macrocystic lesions filled with straw-coloured, protein rich fluid [1,2] and amounts to 4% of all vascular malformations. CL has the potential for extensive infiltration of surrounding tissues making surgical excision difficult and prone to complications [3,4]. CL are also classified into microcystic, macrocystic, and mixed subtypes, according to the size of their cysts [3,5]. It is a benign vascular malformation needs attention because of aesthetic reasons or complications like: local inflammation, infection, sinus formation and haemorrhage [6]. CL are usually found in the head and neck region but their occurrence in salivary gland is very rare [3,6]. Diagnosis and management of salivary gland CL is challenging. Diagnosis is mainly based on history and clinical examinations which is confirmed by dopler study [7]. CT scan and MRI are required in doubtful cases [8], to assess the extent of the lesion of deeper tissue or to study the post treatment response. On review of English literature, CL of salivary gland are very rare with submadibular lesions comprising only 6.25% of all salivary gland CL [2,8,9]. Complete surgical excision is the treatment of choice for CL involving salivary gland [8,9] however excision is difficult [10,11] due to infiltrative nature of these lesions and presence of thin wall of endothelium which can easily tear. Recurrence after macroscopically complete surgical excision is 17% [12,13]. Owing to high recurrence rate and complications after surgery like injury to facial nerve and duct, surgical intervention for CL involving head and neck region is being replaced by sclerotherapy. This study was done to assess the outcome of intralesional bleomycin as sclerotherapy in CL of major salivary glands.
Materials and Methods
This retrospective observational study was done in tertiary care centre of North India in the Department of Paediatric and Plastic surgery. All the children who presented in the Outpatient Department (OPD) with cystic lesion in the parotid or submandibular gland during July 2013 to July 2017 were investigated and all confirmed cases of salivary gland CL were included in the study. Diagnosis was based on history, clinical examination, ultrasound imaging and nature of the aspirated fluid. Fine Needle Aspiration Cytology (FNAC) of the lesion was not done.
Patients who had haemangiomatous component or had significant blood flow in the lesion suggestive of other vascular malformation were excluded from the study. The patients who already received any previous treatment or had history of recurrent infection in the lesion were also excluded. In total seven patients were included in the study.
The steps followed at present centre for management of cystic lymphangioma other than intra-abdominal or intra-thoracic were as followed: (i) After clinical diagnosis [Table/Fig-1] lesion is identified on Ultrasonography (USG) [Table/Fig-2]; (ii) Placement of canula with stent was confirmed on USG; (iii) Fluid was aspirated till lesion collapsed [Table/Fig-3]; (iv) Reconstituted bleomycin solution was injected in the lesion @ 0.5IU/KG and 3IU/mL of the drug was delivered into the cavity under USG Guidance to ensure the delivery of drug in the lesion and avoiding extravasations of the drug; (v) Patients were followed-up with USG for the change in size and specially the volume of the lesion [Table/Fig-4] at 3, 6 and 9 month for first year and 6 monthly for next 2 years.
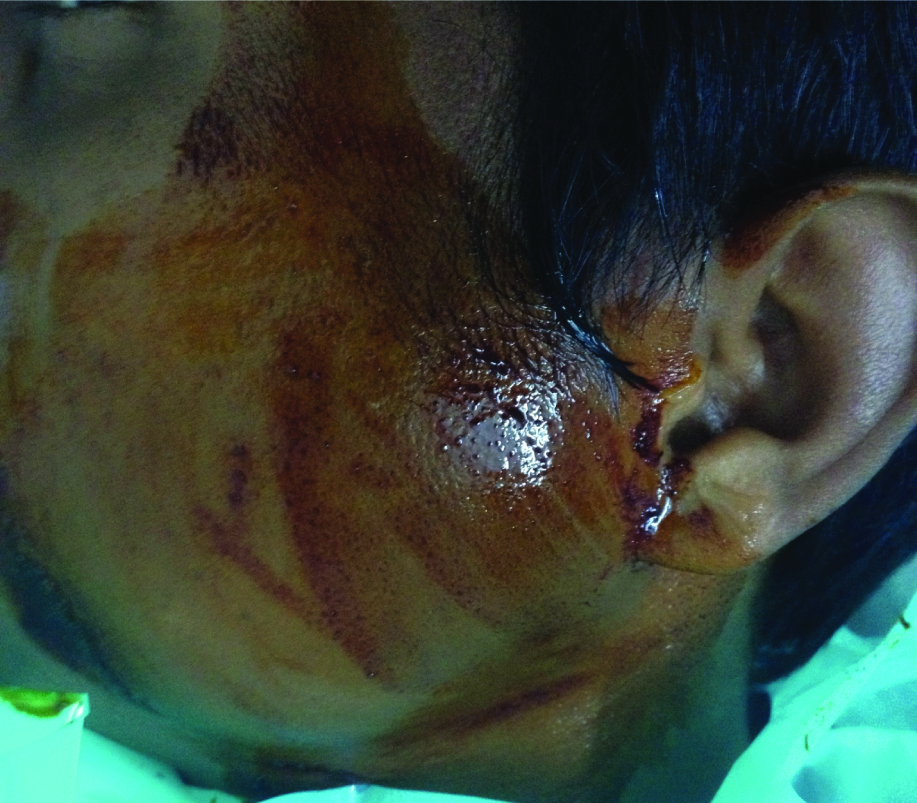
Showing swelling in left parotid area.
Ultrasound of the same showing cystic lesion.
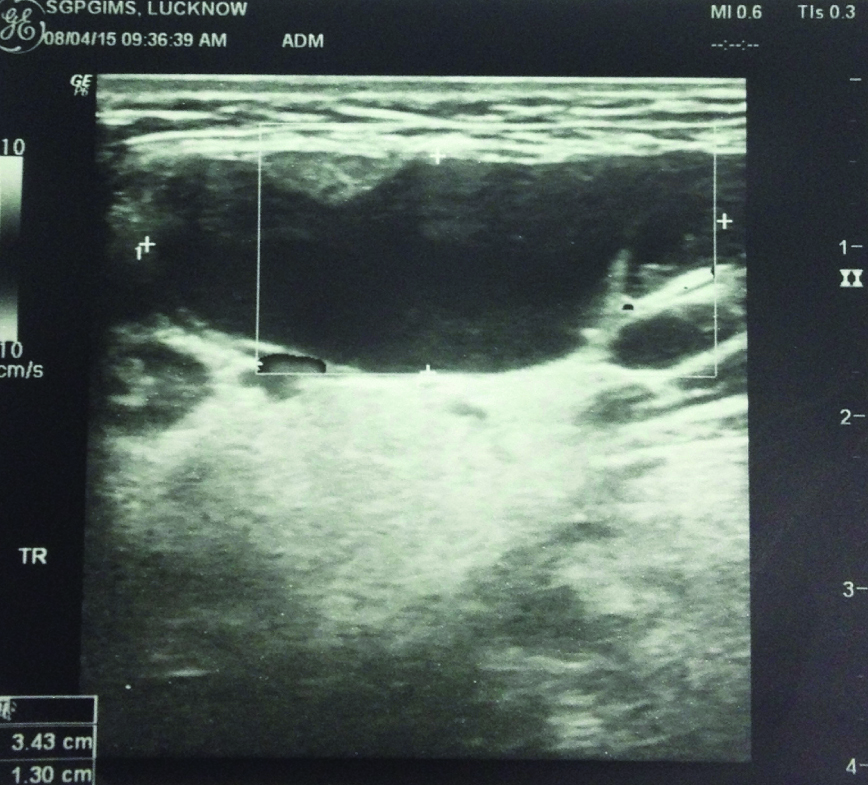
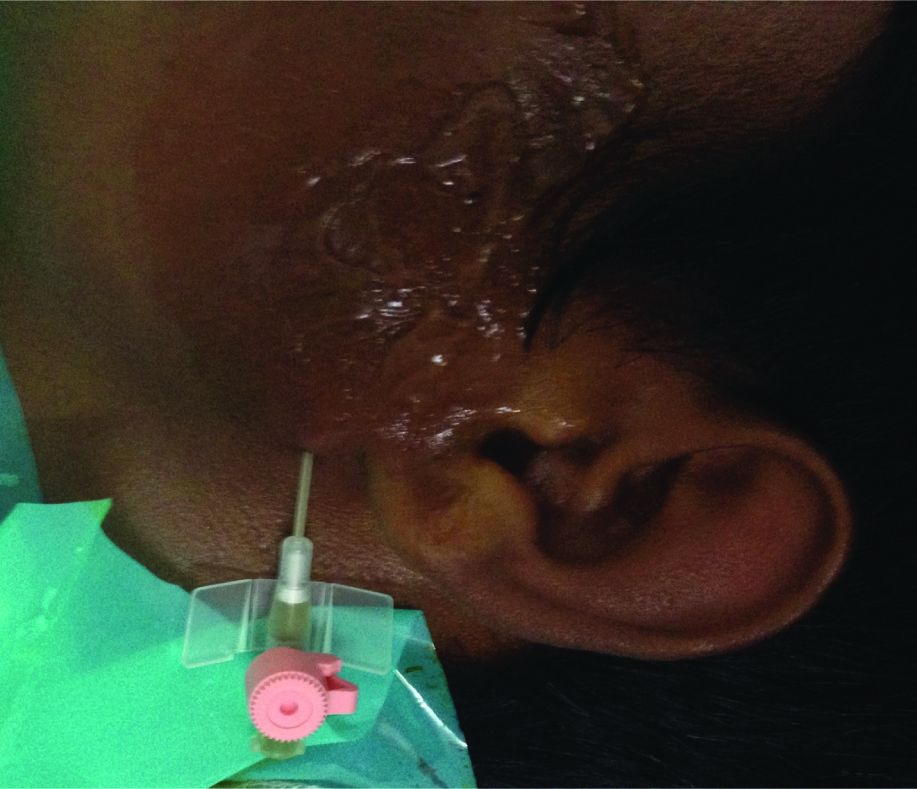
Figure showing placement of cannula for aspiration of the cystic fluid.
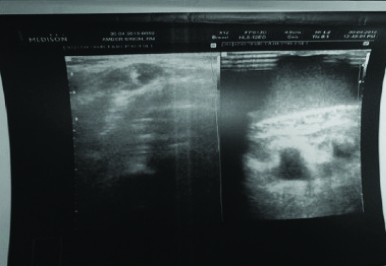
Post sclerotherapy ultrasound showing adequate outcome.
Clinical findings and dopler ultrasound were analysed at each follow-up and repeats session was done at 6 month interval if required. The response was classified as favourable (more than 90% reduction in volume of lesion), partial (reduction of 10-90% of the lesion), or unfavourable (less than 10% reduction of the lesion [14]. The volume was assessed by dopler ultrasound.
Results
Four were male and three were female out of the seven cases. Age ranged from 2 months to 17 years and median age of presentation was 60 months. Parotid gland was involved in four cases, submandibular gland area in two cases and one case had lesion involving both the gland [Table/Fig-5]. Four cases had good response and do not required further surgical intervention. In two cases there was unfavourable response even after second session, hence, were managed surgically. One case had partial response after first session and was later managed surgically. No major complication was observed in present series [Table/Fig-5].
Showing the details of the patients depicting their age, site of lesion, cyst characteristics with response and complications.
ILS: Intralesional sclerotherapy
| Serial no. | Age of patient | Site of cyst and presentation | Number of cyst and volume at time of first session and nature of fluid | Session of ILS with bleomycin | Complication | Response |
|---|
| 1 | 2 month male | Left parotid swelling only | Single large cyst, volume around 10 mL Aspirated fluid was chylous in nature | Single | Mild fever and pain subsides by itself | Clinically no obvious lesion seen, on ultrasound small cystic: not amenable for ILS no further treatment required |
| 2 | 5 month male | Left submandibular area swelling only | Single large cyst with volume around 15-20 mL clear fluid | Single | None | Lesion not appreciated clinically, ultrasound showed almost complete resolution of the lesion with small hypodense residual lesion fibrotic on palpation was removed surgically |
| 3 | 1 year male | Right parotid area and submandibular area (large swelling) | Two large cyst with few small cysts volume including both large cyst around 10 mL clear fluid | Single | Fever and excessive cry needed analgesics for 3 days | More than 90% of resolutin with no apparently visible lesion. No further treatment required |
| 4 | 5 year female | Left submandibular area presented with swelling in the area and fever, pain | 3 macrocyst and few microcystTurbid fluid (history of infection present) | Two | None | More than 90% of resolution with small cystic lesion which was almost completely resolved after 2nd session of ILS with small fibrotic mass No further treatment required |
| 5 | 7 year female | Right parotid region swelling only | 2-3 macrocyst with very small amount | Two | Fever, pain, redness in the region | Non responder, managed surgically |
| 6 | 9 year female | Left parotid area redness in the region, few and welling | 2 macrocyst with few microcyst (history of infection present) | Two | Fever pain subsided after oral analgesic | Lesion was almost unchanged after second session and was managed with surgery |
| 7 | 17 year male | parotid fever and swelling over concerned area | 1 macrocsyts with multiple microcsyts (History of infection present) | Two | Pain, local inflammatory changes fever, | Minimal change after first sessions (less than 20% reduction in size), ultrasound done after second session showed micocsyts which were not amenable for aspiration: considered as nonresponder and was treated surgically |
Statistical Analysis
Data was entered in MS excel and scrutinised for error. For continuous parameter like age, median with minimum and maximum was calculated. Further in case of categorical value, frequency as a percentage was calculated using RStudio software (Inc., Boston, MA URL http://www.rstudio.com/).
Discussion
Most favoured theory for lymphangioma is “dysplastic lymphatic tissue is sequestered in a target tissue during the fetal development”. According to this theory, 6 lymphatic sacs (2 jugulars, 2 iliac, 1 root of mesentery and 1 abdominal aorta) develop in 8th week of gestation, in due course of time, these sac establish connection with each other and finally with venous system of the body. Failure of these sacs, to form connection results in formation of CL [15].
In typical lesion of CL, larger cysts are usually located in periphery whereas smaller one occupies central and/or deeper part of the lesion. These smaller cysts usually infiltrates into the neighboring structures [16]. On histology, they are characterised by single layer endothelium containing; clear watery (lymph) or serous or chylous fluid. CL are usually soft, brilliantly transilluminant, compressible, non-tender and benign in nature [17].
Involvement of salivary gland is uncommon hence, its management is a challenge. Only few cases of CL of parotid [6,7,15-21], or submandibular glands [2] have been reported in adults. Moreover the literature in paediatric patients is very scanty [10,22].
Diagnosis of CL at uncommon sites requires adequate imaging for confirmation. These lesions are evaluated by doppler ultrasound, CT and MRI. Ultrasound is useful for diagnosing superficial lesions but is poor in determining the exact extension into deep structures. The deep extension and infiltration into surrounding tissues can be confirmed by MRI. MRI also helps in differentiating CL from other cystic neoplastic lesion salivary glands. Usually, they present as asymptomatic masses, may present with pain when they are infected and rarely with facial nerve palsy [10]. In most of the reported series, diagnosis of CL was either made on FNAC [16,23,24] or on histopathology of surgically excised tissue [4,20]. In this series, clinical features along with doppler ultrasound and aspiration of chylous or straw coloured fluid were cornerstone to confirm the diagnosis. CL of submandibular gland can be confused with giant or plunging ranula especially in neonates [10] and children [20,21]. In cases of ranula, content is usually mucinous and being a pseudocyst lack the epithelial lining [25]. Radiologically, CL can be differentiated from ranula by the virtue of the fact that CL are characteristically infiltrative in nature and do not respect fascial planes, typically involving contiguous anatomic regions in the neck.
Standard treatment for CL of any region is complete excision [12,24,26]. The presence of important structures in vicinity of CL and infiltration into surrounding tissue planes makes dissection difficult and also there is high recurrence rate, infection, and nerve damage (marginal division of mandibular nerve in submandibular gland and facial nerve in parotid gland). Enucleation of the CL within the gland is difficult due to infiltrative nature of the lesion and presence of thin walled entothelium. Hence, in most cases, surgery involves either a partial or complete excision of the salivary gland leading to loss of function and risk of damage to adjacent nerves. Intralesional sclerotherapy in contrast preserves the salivary gland and the surrounding vital structures while selectively targeting the CL, hence sclerotherapy can be considered as a viable option [14,27-30].
Bleomycin acts on the endothelial lining of the cyst, produces intense tissue reaction and eliminates the secretary function of the endothelial cells [13] where as other sclerosants like polidocanol, ethanol and hypertonic saline act intra as well as peri-lesionally and leads to inflammatory reaction in cyst and surrounding tissue but this complication can be avoided, if Bleomycin is used as sclerosant [13]. This property makes Bleomycin an ideal drug to be used in lesion located near vital structure like major nerve and vessels. In present study, every effort was taken to target each and every cyst which was amenable to aspiration, under USG guidance fluid was aspirated from individual cysts, till it collapsed and then delivery of drug was ensured into the individual cyst and then site is compressed by dressing. Complete aspiration of fluid from cyst, higher concentration of drug (that is 3 IU/mL with in permissible limit of 0.5 IU/KG body weight) and compression on the site, increased the contact time of the drug on endothelial lining of the cyst is the possible explanation for better result in present study.
Although the intralesional sclerotherapy with bleomycin had gained acceptability for management of head and neck macrocystic lymphatic malformation, its role in the management of lymphangioma of salivary gland lesion was not clear. The present study has shown that bleomycin can be used as safe and effective sclerosing agent for CL of major salivary gland. It not only treats the lesion but, also preserve the function of the glandular tissue and peri glandular vital structure.
Limitation(s)
The present study although a novel therapeutic intervention has some limitations. The small sample size, lack of comparison with other treatment modalities and absence of randomisation are the major shortcoming of this study. A well randomised study to compare the outcome of surgery verses sclerotherapy CL involving major salivary gland will be more effective in making conclusive remark i.e., that sclerotherapy can be a first line treatment for these lesion.
Conclusion(s)
Based on the experience of intralesional bleomycin, which is the first line treatment modality for head and neck CL, this should also be the first line treatment for CL involving the major salivary gland. Bleomycin sclerotherapy delivered under ultrasound guidance with direct puncture of the individual cysts can provide a safe, easy and reproducible treatment modality for the management of this rare entity though larger study and trial is required for definitive conclusion.