Comparative Evaluation of Antimicrobial Efficacy of Sodium Hypochlorite, Silver Diamine Fluoride, Chitosan and Bioactive Glass Nanoparticles as Root Canal Irrigants against the Bacterial Strain of Enterococcus faecalis- An In vitro Study
Dimple Vijay Chaudhari1, ND Shashikiran2, Ankita Maurya3, Sachin Gugwad4, Namrata Gaonkar5, Swapnil Taur6, Savita Hadakar7
1 Dental Student, Department of Dentistry, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
2 Dean and Head, Department of Dentistry, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
3 Postgraduate Student, Department of Paedodontics, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
4 Associate Professor, Department of Paedodontics, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
5 Associate Professor, Department of Paedodontics, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
6 Assistant Professor, Department of Paedodontics, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
7 Assistant Professor, Department of Paedodontics, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: ND Shashikiran, Department of Paedodontics and Preventive Dentistry, School of Dental Sciences, KIMSDU, Karad, Maharastra, India.
E-mail: drndshashikiran@gmail.com
Introduction
Enterococcus faecalis (E. faecalis) is the most commonly encountered microorganism detected in persistent root canal infections. These bacteria possess certain virulence factors, invade dentinal tubules and resist nutritional deprivation. Proper irrigation is an essential step for success in root canal therapies which is achieved by using excellent endodontic irrigants.
Aim
To evaluate the antimicrobial efficacy of Sodium Hypochlorite (NaOCl), Silver Diamine Fluoride (SDF), Chitosan Nanoparticles (CNPs) and Bioactive Glass Nanoparticles (BAGNP) as root canal irrigants against the bacterial strain of Enterococcus Faecalis (E. faecalis) using agar well diffusion method.
Materials and Methods
In this in-vitro study, the test materials were manipulated in accordance with the manufacturer’s instructions. The antimicrobial properties of root canal irrigants were evaluated by using agar diffusion method using bacterial strain of Enterococcus faecalis (ATCC 29212). 0.25 mL of each irrigant was placed on to 6.5 mm diameter blotting papers which were placed in 7 mm diameter wells on the Mueller Hinton agar plates. Later, E. faecalis strains were inoculated with sterile cotton swab on to the agar plates. All the plates were incubated at 37°C and evaluated at 24, 48 and 72 hours after pre-diffusion of test materials for two hours at room temperature. A 0.5 mm precision ruler was used to measure the microbial inhibition zones and then the results were expressed as mean and standard deviation. The results were tabulated and analysed using One-way ANOVA to find out if there was a significant overall difference between the mean zones of inhibition of various irrigants and Post-hoc Tukey HSD test to find out where the differences occurred. The p-value <0.05 was considered as significant.
Results
Sodium Hypochlorite showed the greatest zone of inhibition followed by SDF, Bioactive Glass Nanoparticles and Chitosan Nanoparticles respectively (p<0.0107). Mean zones of inhibition for Sodium Hypochlorite, SDF, Bioactive Glass Nanoparticles and Chitosan Nanoparticles were 11.6 mm, 6.6 mm, 5 mm, and 1.4 mm, respectively. There were no observable differences on 24, 48 and 72 hours.
Conclusion
Sodium Hypochlorite was the most effective root canal irrigant followed by SDF and Bioactive Glass Nanoparticle whereas Chitosan Nanoparticles was the least efficacious compared to the rest against Enterococcus Faecalis.
Disc diffusion, Endodontics, Re-infection, Zone of inhibition
Introduction
Enterococcus faecalis (E. faecalis) is one of the most commonly detected and isolated microorganisms from teeth with pulpal necrosis [1]. As the host defence mechanisms cannot eliminate the bacteria, they must be handled by means of chemical and mechanical debridement approaches consisting of irrigation of root canal with chemical agents [2]. Bacteria are found in highly complex and organized biofilms adhering to the canal walls or they predominate in areas of complex anatomy such as apical constriction and lateral walls which make them inaccessible during certain root canal procedures. Specific treatment strategies which include effective irrigation of root canals and show promising antibacterial results are needed to overcome such limitations and to prevent failure of root canal treatments [3]. Chemo-mechanical debridement of the root canal, which includes mechanical instrumentation and application of chemical cleaning, is the main procedure followed for eliminating canal bacteria [4]. All endodontic infections are poly-microbial in nature with differences between the types of micro-organisms isolated from primary and secondary root canal infections [5,6]. The micro-organism E.faecalis is an anaerobic gram-positive coccus that can tolerate harsh environmental conditions, including high alkaline pH, dry climate and high concentration of salts [7]. The use of root canal irrigants has proved to be a critical treatment strategy in endodontic therapy as they aid in disinfecting and lubricating the root canal, flushing out particles from the canal, and dissolving organic and inorganic tissues [8]. Sodium Hypochlorite (NaOCl) is the medicament of choice due to its ability to dissolve organic substances present in the root canal system, its affordability, its efficacy against pathogenic organisms like E. faecalis and pulp digesting property in endodontic therapy [9]. Sodium hypochlorite is used in non-surgical endodontic treatment as a powerful antimicrobial agent for its chemical dissolution properties and as a lubricant during instrumentation [10]. Silver Diamine Fluoride (SDF), is an anti-cariogenic agent, that is deemed to be very powerful as an antimicrobial root canal irrigant and for inter-appointment dressing specifically in pediatric dentistry [11,12]. Chitosan is a polymer with wide range of application in the medical field. It is either in part or completely de-acetylated chitin. Fungal cell walls and crustacean shells, for example, contain chitin naturally, it is completely biodegradable and biocompatible and may be used as an adhesive and wound dressing material [13]. Chitosan has been investigated as an antimicrobial material against target organisms like algae, micro-organism (gram positive and gram negative), Chitosan has been investigated as an antimicrobial material in various in-vitro and in-vivo experiments designed to study interactions with chitosan against target organisms like algae, gam positive and gram negative bacteria and fungi such as yeasts [14].
In an aqueous environment bioactive glasses release calcium and phosphate ions which result in an increased pH and osmotic pressure of the surroundings thus exerting an antimicrobial effect [15]. Shrestha A et al., and Kishen A et al., have showed that E. faecalis pathogens present in a planktonic state can be completely eliminated with Bioactive Glass and a significant reduction of bacteria in the biofilm state can be achieved [16,17].
To the best of our knowledge, this is the first time this combination of irrigants is being tested and compared for their anti-microbial efficacy against E. faecalis.
Hence, this study was carried out to evaluate the antimicrobial efficacy of various root canal irrigants like Sodium hypochlorite, SDF, Chitosan Nanoparticles (CNPs) and Bioactive Glass Nanoparticles (BAGNP) against E. faecalis bacteria in-vitro using disc diffusion method and the relative efficacies were recorded after 24, 48 and 72 hours of incubation.
Materials and Methods
This was an in -vitro study conducted in (August 2019) at the Department of Microbiology, Krishna Institute of Medical Sciences Deemed University, Karad, Maharashtra. The study was approved by the Institutional Ethics Committee under the protocol number 0220/2018-19. Reference number (KIMSDU/IEC/08/2018).
Sampling Technique
Randomised sampling technique was carried out for sample size determination for minimum number of petri plates required using the following formula:
n= (z2α/2 p×q)/d2 [18]
Where, n=sample size
z=statistics corresponding to level of confidence
p=expected prevalence
d=precision or margin of error
q=calculated from prevalence
The values, p=0.90, d=5%, α=0.05, zα/2=1.64
Therefore, the minimum sample size required was determined to be 10 after substituting the values in the above formula. Hence, the total number of petri dishes for the study equalled to 10.
The antimicrobial property of root canal irrigants was evaluated by agar diffusion method [19] using strains of Enterococcus faecalis (ATCC 29212). Primary isolation was done on Mueller Hinton agar plates and magenta pink-coloured colonies were grown.
In the present study, four endodontic irrigants were used i.e., 2.5% Sodium Hypochlorite, 38% SDF, Bioactive glass Nanoparticles (1 g/mL in distilled water) and Chitosan Nanoparticles (1 mg/mL in 0.1% acetic acid). The test materials were manipulated in accordance with the manufacturer’s instructions. Solution of 2.5% Sodium hypochlorite and 38% SDF were used which were commercially available. Solution of Chitosan Nanoparticles was prepared by diluting 1 mg/mL in 0.1% Acetic acid [20]. Bioactive Glass Nanoparticle based on 70SiO2-26CaO-4P2O5 (weight %) was synthesised by a modified Sol-gel method [21]. To synthesise the bioglass nanoparticles via modified sol-gel method, first tetraethyl orthosilicate was added drop by drop to a mixture containing distilled water, ethanol and 2 mole nitric acid followed by stirring for 1 hour to achieve the complete acid hydrolysis of Tetraethyl orthosilicate. Triethyl phosphate and Calcium nitrate tetrahydrate were added sequentially while stirring the solution. Then the whole solution was placed in an ultrasonic bath working at a frequency of 50-60 kilohertz, 100/200 Watt. 2 Mole ammonia solution was added drop by drop to the mixture while mechanically stirring it to prevent the formation of a bulk gel. The sol was completely transformed to a white gel within few minutes. Finally, the prepared gel was kept for drying at 75°C for two days in a drying oven and then subjected to heat treatment at 700°C for three hours with a rate of 3°C/min to stabilise and convert such gel to glass. A 1% Solution was prepared by mixing 1g of Bioactive glass Nanoparticle in 1 mL of distilled water [21].
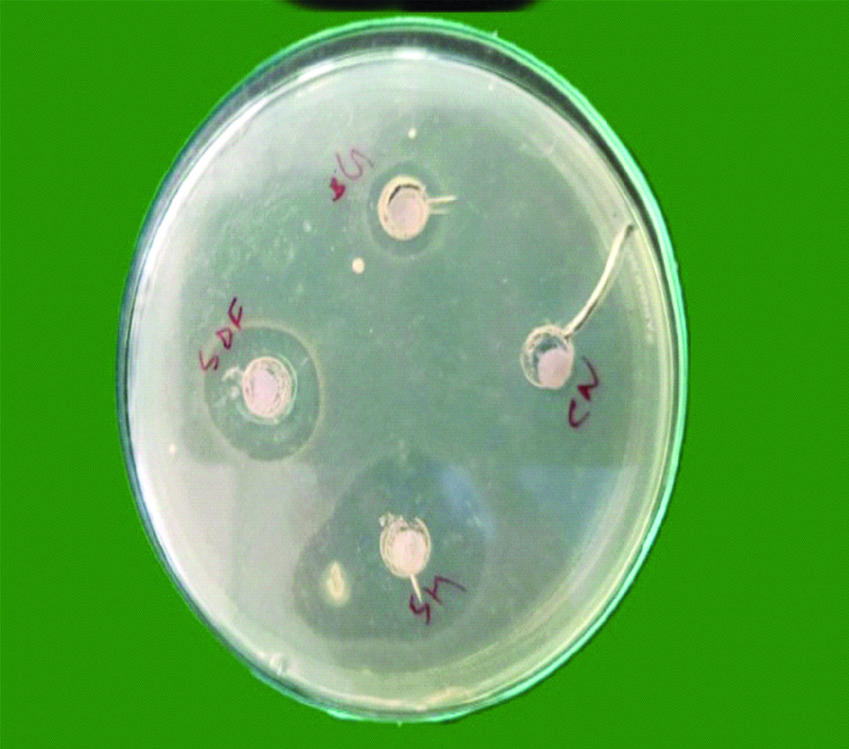
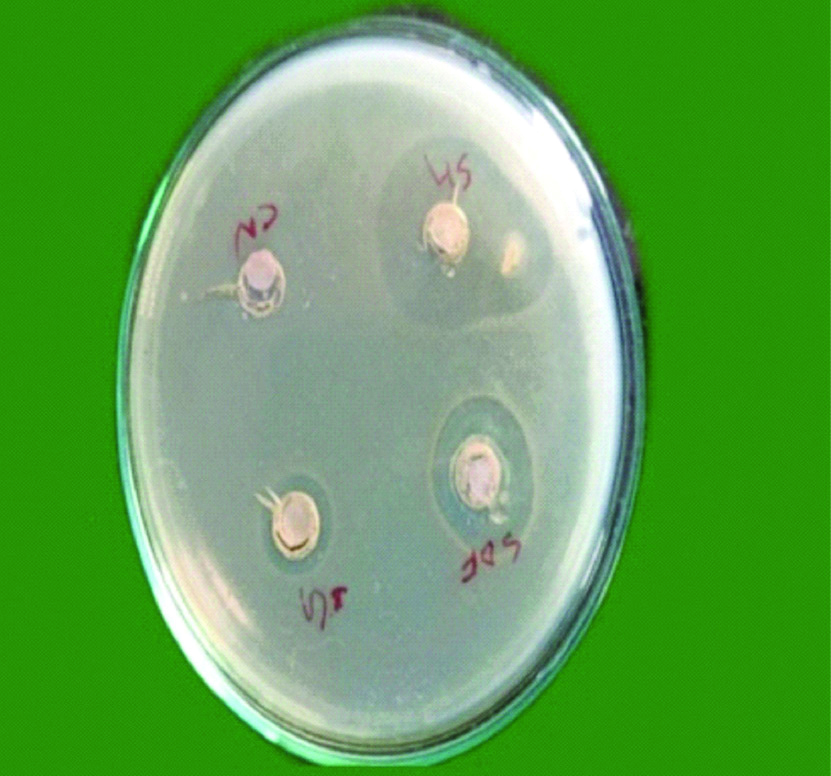
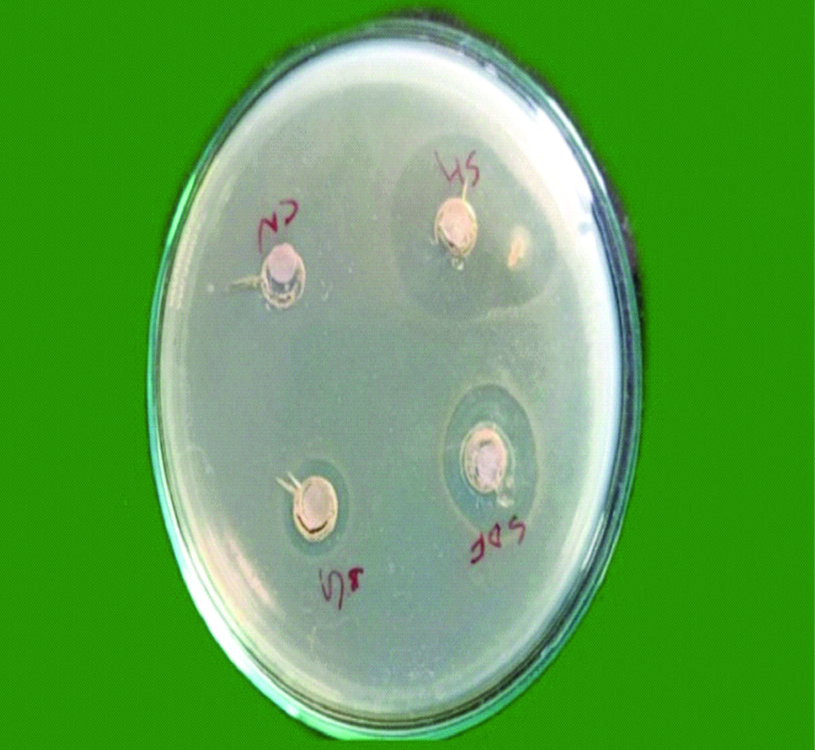
Bacteria were diluted to obtain suspension of approximately 5×106 colony forming units in sterile Trypticase Soy Broth (TSB) obtained by spectrophotometer by forming two layers, one is the seed layer and next is the second layer. Microbial strains were confirmed by colony forming units and growth characteristics. A 10 mL of Mueller Hinton agar was poured in petri plates to form a base layer. When it was solidified, a second layer containing 10 mL of Mueller Hinton agar and 200 μL of microbial standardised suspensions were poured over it. Wells of 7 mm diameter were prepared on Mueller Hinton agar plates using sterile core borer. Followed by this 0.25 mL of each irrigant was placed on to the 6.5 mm diameter blotting paper. The soaked 6.5 mm blotting paper was placed into the wells which was created on the agar plates. Later E. faecalis strains were inoculated with sterile cotton swab on to this agar plate [22]. After pre-diffusion of test materials for two hours at room temperature, all the plates were incubated at 37°C and evaluated at 24, 48 and 72 hours to analyse whether there were any significant changes in zone of inhibition of individual irrigant after 24, 48 and 72 hours [Table/Fig-1,2 and 3], respectively.
Showing microbial zone of inhibition after 24 hours.
SH: Sodium hypochlorite, SDF: Silver diamine fluoride, BG: Bioactive glass nanoparticle, CN: Chitosan nanoparticle.
Showing microbial zone of inhibition after 48 hours.
SH: Sodium hypochlorite; SDF: Silver diamine fluoride; BG: Bioactive glass nanoparticle; CN: Chitosan nanoparticle
Showing microbial zone of inhibition after 72 hours.
SH: Sodium hypochlorite; SDF: Silver diamine fluoride; BG: Bioactive glass nanoparticle; CN: Chitosan nanoparticle
Generation time is the time duration for any bacteria to become double by binary fission which was 72 hours for E. faecalis [23]. In case of E. faecalis, the growth usually comes after 24 hours, but some studies conclude that growth gets completed after 72 hours [24]. So, to confirm the growth and for further confirmation of the results, bacteria were incubated for 72 hours and readings were recorded at 24, 48 and 72 hours. A study verified three-day-old biofilm showing E. faecalis colonising the dentin surface and starting to invade the patent dentinal tubules [24]. A 0.5 mm precision ruler was used to determine the microbial inhibition zones and the results were expressed as the mean and standard deviation. The test results were measured by the author, double blinding was ensured by two other authors to eliminate the bias.
Statistical Analysis
The results were analysed using one-way ANOVA with INSTAT software (GraphPad, San Diego, CA) to find out if there was a significant overall difference between the mean zones of inhibition of various irrigants and Post-hoc Tukey HSD test to find out where the differences occurred. The p-value was considered significant at (p<0.05).
Results
Raw data table representing [Table/Fig-4] zones of inhibition (in mm) of root canal irrigants after 24, 48 and 72 hours of incubation of 10 Petri Plates.
Zones of Inhibition(in mm) of Root Canal Irrigants after 24, 48 and 72 hours of incubation of 10 Petri Plates.
| Sodium hypochlorite | Silver diamine fluoride | Bioactive glass nanoparticles | Chitosan nanoparticles |
|---|
| 24 hours | 48 hours | 72 hours | 24 hours | 48 hours | 72 hours | 24 hours | 48 hours | 72 hours | 24 hours | 48 hours | 72 hours |
|---|
| 11 | 11 | 11 | 8 | 8 | 8 | 6 | 6 | 6 | 0 | 0 | 0 |
| 15 | 15 | 15 | 6 | 6 | 6 | 5 | 5 | 5 | 0 | 0 | 0 |
| 8 | 8 | 8 | 7 | 7 | 7 | 4 | 4 | 4 | 4 | 4 | 4 |
| 16 | 16 | 16 | 8 | 8 | 8 | 5 | 5 | 5 | 0 | 0 | 0 |
| 11 | 11 | 11 | 6 | 6 | 6 | 4 | 4 | 4 | 0 | 0 | 0 |
| 11 | 11 | 11 | 5 | 5 | 5 | 6 | 6 | 6 | 3 | 3 | 3 |
| 12 | 12 | 12 | 7 | 7 | 7 | 4 | 4 | 4 | 0 | 0 | 0 |
| 11 | 11 | 11 | 6 | 6 | 6 | 5 | 5 | 5 | 0 | 0 | 0 |
| 13 | 13 | 13 | 6 | 6 | 6 | 4 | 4 | 4 | 2 | 2 | 2 |
| 8 | 8 | 8 | 7 | 7 | 7 | 7 | 7 | 7 | 5 | 5 | 5 |
Mean zones of inhibition after 24 hours are shown in [Table/Fig-5]. Significant difference was present in the overall test means (p<0.0107). Sodium Hypochlorite showed highest mean zone of inhibition followed by SDF, Bioactive Glass Nanoparticles and Chitosan Nanoparticles. No changes were observed in the zones of inhibition after 24, 48 and 72 hours. [Table/Fig-6] shows the comparison between the root canal irrigants against each other at 72 hours, the results were significant at (p<0.01).
Diameter of inhibition zone around the wells after 24 hours. Data presented as mean and standard deviation.
| Root canal irrigants | Mean | Standard deviation |
|---|
| Sodium hypochlorite | 11.6 | 2.591 |
| Silver diamine fluoride | 6.6 | 0.9661 |
| Bioactive glass nanoparticles | 5 | 1.054 |
| Chitosan nanoparticles | 1.4 | 1.955 |
| F 57.07 | |
| p-value 0.0107* | |
*The p-value <0.05 was considered as significant.
Tests used: One-way ANOVA using INSTAT software (GraphPad, San Diego, CA)
Shows the post-hoc results at 72 hours: Tukey-Kramer Multiple Comparisons Test using standard parametric method
| Comparisons between root canal irrigants | Tukey HSD inference |
|---|
| SDF vs NaOCl | p<0.01** |
| NaOCl vs BAGNP | p<0.01** |
| CNP vs NaOCl | p<0.01** |
| SDF vs BAGNP | p<0.01** |
| CNP vs SDF | p<0.01** |
| CNP vs BAGNP | p<0.01** |
**The p-value <0.05 was considered as significant
Discussion
E. faecalis is the most common bacterial species found in association with endodontically treated teeth with chronic apical periodontitis. The development of calcified biofilm on root canal dentin by E. faecalis can be a potential factor that enables them to persist even after endodontic treatment.
Sodium Hypochlorite is the most commonly used solution in root canal treatments, as it’s a low-cost approach that demonstrates a very powerful antimicrobial activity against microbiota of infected root canals [26]. NaOCl ionises in water into Na+ and the hypochlorite ion, OCl-, establishing equilibrium with hypochlorous acid (HOCl). Chlorine exists predominantly as HOCl at acidic and neutral pH, whereas OCl- predominates at pH of 9 and above. Hypochloric acid is responsible for the antibacterial activity [27]. In this study, NaOCl showed significant results in antimicrobial efficacy as compared to other root canal irrigants. It has been approved that 2.5% NaOCl is extremely useful in the removal of vital pulp tissue from dentinal walls [28]. A 1.5-2.5% Sodium Hypochlorite solution as an endodontic irrigant is preferred as the gold standard for root canal cleansing and disinfection [29]. Jaiswal et al., found out that NaOCl was highly effective in eliminating E. faecalis grown in biofilm [30].
SDF is an anti-cariogenic material with a high fluoride release capacity [31]. SDF is very effective as an antimicrobial endodontic irrigant and for dressings given in between the appointments [12,15]. Other root canal irrigants like Sodium Hypochlorite, Bioactive Glass Nanoparticles and Chitosan Nanoparticles considered in this study fail at releasing fluorides which adds to the antimicrobial efficacy. As fluoride has a direct inhibitory effect on the metabolic activity of bacteria (gram positive and gram negative) [32]. The Silver Nanoparticle solution has been endorsed as an alternative to root canal irrigating solutions not only for its effective bactericidal capacity but additionally for its biocompatibility, specifically at lower concentrations [33]. SDF can enhance the hardness of enamel surface and re-mineralise it [12,34]. In this study, statistically significant difference was seen between NaOCl and SDF in inhibitory microbial zones (p<0.01). A previous study noted there was a significant removal of bacterial biofilm with a few residual dead cells near the dentin surfaces using 3.8% SDF [35]. NaOCl and Ag (NH3)2F were found to be powerful against E. faecalis biofilms, and there was no major difference in reduction of microorganisms [35].
Ag (NH3)2F has the ability to be used as an antimicrobial root canal irrigant or inter-appointment dressing, particularly at sites wherein browning/blackening of dentin by using metallic silver isn’t a major problem [20]. In an in -vitro model studied by Prabhakar AR and Kumar SC, BAG was effective against E. faecalis as an intra canal medicament [36]. In this study, BAGNP showed less antimicrobial effect when compared to NaOCl, SDF and showed higher antimicrobial efficacy than CNPS.
CNPs have the ability to be used as the last irrigant in comparison to Ethylenediaminetetraacetic Acid (EDTA) in root canal treatment due to their potential to behave as an antibacterial and a chelating agent on root dentin [37]. Yadav P et al., proved the anti-bacterial efficacy of Chitosan to be almost equivalent to 3% NaOCl, suggesting that CNPs can be used as an endodontic irrigant to overcome the deleterious consequences, concentration and time dependent outcomes of the conventional irrigants like NaOCl and Chlorhexidine on dentine [22]. According to this study, CNP showed less antibacterial efficacy when compared to other irrigants. Results disagreement may be due to different concentration of Chitosan solution, discrepancy in tested strains, variances in methodology, and may be because of different incubation conditions.
Limitation(s)
In-vitro studies have the limitation of laboratory and clinical setup errors. Contamination of the irrigants with other biofilms and fluids in oral cavity may alter the results. The microorganisms of root canal other than E. faecalis may show unpredictable reactions to the studied irrigants. This study has exclusively used E. faecalis strain ATCC29212. In-vitro studies cannot re-multiply the microorganism which is the possibility in host in-vivo. Agar depth can have an effect on the accuracy of plate-based assays and the probable reason for the same could be antimicrobial agent diffusion. Therefore, further clinical studies are indicated.
Conclusion(s)
Within the limitations of this study, it can be concluded that Sodium Hypochlorite showed better results as compared to the other irrigants in the following order of decreasing antimicrobial efficacy against E. faecalis: SDF, Bioactive Glass Nanoparticles and Chitosan Nanoparticles. Therefore, it is safe to say that Sodium Hypochlorite is still the most effective ‘gold standard irrigant’. No decrease in zone of inhibition after 72 hours proved that the efficacy of the root canal irrigants is maintained at least till three days. Further studies should be carried out for longer period to substantiate findings of this study.
*The p-value <0.05 was considered as significant.
Tests used: One-way ANOVA using INSTAT software (GraphPad, San Diego, CA)
**The p-value <0.05 was considered as significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 05, 2020
Manual Googling: Apr 22, 2020
iThenticate Software: Apr 29, 2020 (19%)
[1]. Rodríguez-Niklitschek C, Clinical implications of Enterococcus faecalis microbial contamination in root canals of devitalized teeth: Literature reviewRevista Odontológica Mexicana 2015 19(3):181-86.10.1016/j.rodmex.2015.04.002 [Google Scholar] [CrossRef]
[2]. Ghivari SB, Bhattacharya H, Bhat KG, Pujar MA, Antimicrobial activity of root canal irrigants against biofilm forming pathogens-An in vitro studyJ Conserv Dent 2017 20(3):14710.4103/JCD.JCD_38_1629279615 [Google Scholar] [CrossRef] [PubMed]
[3]. Blue CM, Darby’s Comprehensive Review of Dental Hygiene-E-BookElsevier Health Sciences 2015 8th edMosby [Google Scholar]
[4]. Reyhani MF, Rezagholizadeh Y, Narimani MR, Rezagholizadeh L, Mazani M, Barhaghi MH, Antibacterial effect of different concentrations of sodium hypochlorite on Enterococcus faecalis biofilms in root canalsJ Dent Res Dent Clin Dent Prospects 2017 11(4):215-21. [Google Scholar]
[5]. Sundqvist G, Figdor D, Life as an endodontic pathogen: Ecological differences between the untreated and root-filled root canalsEndodontic Topics 2003 6(1):03-28.10.1111/j.1601-1546.2003.00054.x [Google Scholar] [CrossRef]
[6]. Gomes BP, Pinheiro ET, Gadê CR, Sousa EL, Ferraz CC, Zaia AA, Microbiological examination of infected dental root canalsOral Microbiol Immunol 2004 19(2):71-76.10.1046/j.0902-0055.2003.00116.x14871344 [Google Scholar] [CrossRef] [PubMed]
[7]. Jhajharia K, Parolia A, Shetty KV, Mehta LK, Biofilm in endodontics: A reviewJ Int Soc Prev Community Dent 2015 5(1):01-12.10.4103/2231-0762.15195625767760 [Google Scholar] [CrossRef] [PubMed]
[8]. Borzini L, Condò R, De Dominicis P, Casaglia A, Cerroni L, Root canal irrigation: Chemical agents and plant extracts against Enterococcus faecalisOpen Dent J 2016 10:692-703.10.2174/187421060161001069228217184 [Google Scholar] [CrossRef] [PubMed]
[9]. Torabinejad M, Root canal irrigants and disinfectantsEndodontics: Colleagues for Excellence 2011 :02-07. [Google Scholar]
[10]. Iqbal A, Antimicrobial irrigants in the endodontic therapyInt J Publ Health Sci 2012 6(2):186-92.10.12816/0005998 [Google Scholar] [CrossRef]
[11]. Yamaga R, Yokomizo I, Arrestment of caries of deciduous teeth with diamine silver fluorideDental Outlook 1969 33:1007-13. [Google Scholar]
[12]. Chu CH, Lo EC, Lin HC, Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school childrenFront Dent 2002 81(11):767-70.10.1177/15440591020810110912407092 [Google Scholar] [CrossRef] [PubMed]
[13]. Roy GS, Shirsat SN, Mishra CA, Waghulde OS, Kale KM, A review on chitosan nanoparticles applications in drug deliveryJ Pharmacogn Phytochem 2018 7(6S):01-04.10.22271/phyto.2018.v7.isp6.1.01 [Google Scholar] [CrossRef]
[14]. Goy RC, Britto DD, Assis OB, A review of the antimicrobial activity of chitosanPolímeros 2009 19(3):241-47.10.1590/S0104-14282009000300013 [Google Scholar] [CrossRef]
[15]. Stoor P, Söderling E, Salonen JI, Antibacterial effects of a bioactive glass paste on oral microorganismsBiomaterInvestig Dent 1998 56(3):161-65.10.1080/0001635984229019688225 [Google Scholar] [CrossRef] [PubMed]
[16]. Shrestha A, Zhilong S, Gee NK, Kishen A, Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activityEur Endod J 2010 36(6):1030-35. [Google Scholar]
[17]. Kishen A, Shi Z, Shrestha A, Neoh KG, An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfectionEur Endod J 2008 34(12):1515-20.10.1016/j.joen.2008.08.03519026885 [Google Scholar] [CrossRef] [PubMed]
[18]. Lachin JM, Introduction to sample size determination and power analysis for clinical trialsControl Clin Trials 1981 2(2):93-113.10.1016/0197-2456(81)90001-5 [Google Scholar] [CrossRef]
[19]. Luddin N, Ahmed HM, The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methodsJ Conserv Dent 2013 16(1):09-16.10.4103/0972-0707.10529123349569 [Google Scholar] [CrossRef] [PubMed]
[20]. Mathew VB, Madhusudhana K, Sivakumar N, Venugopal T, Reddy RK, Anti-microbial efficiency of silver diamine fluoride as an endodontic medicament-An ex vivo studyContemp Clin Dent 2012 3(3):262-64.10.4103/0976-237X.10361523293478 [Google Scholar] [CrossRef] [PubMed]
[21]. El-Kady AM, Farag MM, Bioactive glass nanoparticles as a new delivery system for sustained 5-fluorouracil release: Characterization and evaluation of drug release mechanismJ Nanomater 2015 16(1):83920710.1155/2015/839207 [Google Scholar] [CrossRef]
[22]. Yadav P, Chaudhary S, Saxena RK, Talwar S, Yadav S, Evaluation of antimicrobial and antifungal efficacy of chitosan as endodontic irrigant against enterococcus faecalis and candida albicans biofilm formed on tooth substrateJ Clin Exp Dent 2017 9(3):e36110.4317/jced.5321028298975 [Google Scholar] [CrossRef] [PubMed]
[23]. Daw K, Baghdayan AS, Awasthi S, Shankar N, Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitroFEMS Immunol Med Microbiol 2012 65(2):270-82.10.1111/j.1574-695X.2012.00944.x22333034 [Google Scholar] [CrossRef] [PubMed]
[24]. Saber SE, El-Hady SA, Development of an intracanal mature Enterococcus faecalis biofilm and its susceptibility to some antimicrobial intracanal medications; An in vitro studyInt J Oral Implantol (New Malden) 2012 6(01):043-50.10.1055/s-0039-169892922229006 [Google Scholar] [CrossRef] [PubMed]
[25]. Jaiswal N, Sinha DJ, Singh UP, Singh K, Jandial UA, Goel S, Evaluation of antibacterial efficacy of chitosan, chlorhexidine, propolis and sodium hypochlorite on Enterococcus faecalis biofilm: An in vitro studyClin Exp Dent Res 2017 9(9):e106629075407 [Google Scholar] [PubMed]
[26]. Bosch-Aranda ML, Canalda-Sahli C, Figueiredo R, Gay-Escoda C, Complications following an accidental sodium hypochlorite extrusion: A report of two casesClin Exp Dent Res 2012 4(3):e19410.4317/jced.5076724558554 [Google Scholar] [CrossRef] [PubMed]
[27]. Gusiyska A, Gyulbenkiyan E VR, Dyulgerova E, Mironova J, Effective root canal irrigation-a key factor of endodontic treatment review of the literatureInt J Recent Sci Res 2016 7(4):9962-70. [Google Scholar]
[28]. Aranda-Garcia AR, Guerreiro-Tanomaru JM, Faria-Júnior NB, Chavez-Andrade GM, Leonardo RT, Tanomaru-Filho M, Antibacterial effectiveness of several irrigating solutions and the Endox Plus system-An ex vivo studyInt Endod J 2012 45(12):1091-96.10.1111/j.1365-2591.2012.02069.x22554197 [Google Scholar] [CrossRef] [PubMed]
[29]. Zehnder M, Root canal irrigantsEur Endod J 2006 32(5):389-98.10.1016/j.joen.2005.09.01416631834 [Google Scholar] [CrossRef] [PubMed]
[30]. Jaiswal N, Sinha DJ, Singh UP, Singh K, Jandial UA, Goel S, Evaluation of antibacterial efficacy of chitosan, chlorhexidine, propolis and sodium hypochlorite on Enterococcus faecalis biofilm: An in vitro studyClin Exp Dent Res 2017 9(9):e106610.4317/jced.5377729075407 [Google Scholar] [CrossRef] [PubMed]
[31]. Silveira LF, Silveira CF, Martos J, de Castro LA, Evaluation of the different irrigation regimens with sodium hypochlorite and EDTA in removing the smear layer during root canal preparationJ Microsc Ultrastruct 2013 1(1-2):51-56.10.1016/j.jmau.2013.06.003 [Google Scholar] [CrossRef]
[32]. Nouri MR, Titley KC, Paediatrics-A Review of the Antibacterial Effect of FluorideOral Health 2003 93(1):08-12. [Google Scholar]
[33]. Rai MK, Deshmukh SD, Ingle AP, Gade AK, Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteriaJ Appl Microbiol Biochem 2012 112(5):841-52.10.1111/j.1365-2672.2012.05253.x22324439 [Google Scholar] [CrossRef] [PubMed]
[34]. Gomes-Filho JE, Silva FO, Watanabe S, Cintra LT, Tendoro KV, Dalto LG, Tissue reaction to silver nanoparticles dispersion as an alternative irrigating solutionEur Endod J 2010 36(10):1698-702.10.1016/j.joen.2010.07.00720850681 [Google Scholar] [CrossRef] [PubMed]
[35]. Al-Madi EM, Al-Jamie MA, Al-Owaid NM, Almohaimede AA, Al-Owid AM, Antibacterial efficacy of silver diamine fluoride as a root canal irrigantClin Exp Dent Res 2019 5(5):551-56.10.1002/cre2.22231687190 [Google Scholar] [CrossRef] [PubMed]
[36]. Prabhakar AR, Kumar SC, Antibacterial effect of bioactive glass in combination with powdered enamel and dentinIndian J Dent Res 2010 21(1):3010.4103/0970-9290.6280720427903 [Google Scholar] [CrossRef] [PubMed]
[37]. del Carpio-Perochena A, Bramante CM, Duarte MA, de Moura MR, Aouada FA, Kishen A, Chelating and antibacterial properties of chitosan nanoparticles on dentinRestor Dent Endod 2015 40(3):195-201.10.5395/rde.2015.40.3.19526295022 [Google Scholar] [CrossRef] [PubMed]