Neoplasms and tumour like lesions that originate from chest wall tissues are infrequent compared with tumours in other parts of the body and acquaintance with these disease entities is important for their diagnosis. Primary chest wall tumours are classified on the basis of tissue of origin [1,2] benign versus malignant [3,4]. Of all intrinsic bone tumours, primary neoplasms of bony chest have been reported to comprise 7 to 8% [5,6]. Tumours that arise from the chest wall include from the bony structures (sternum, scapula, ribs) or from the adjacent soft tissue.
The objectives of this study, was to describe the distribution of different clinical profiles and radiological presentation of chest wall lesions and make correlation and analysis of their histological findings, along with confirmation of the diagnosis with Immunohistochemistry (IHC) wherever necessary.
Materials and Methods
This was a prospective study conducted from December 2017 to December 2019 at Department of Pathology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal (India) and comprised of 16 cases. The patients presented with chest mass, pain, dyspnea. All the patients were investigated with complete haemogram, Chest X-ray (CXR), Computed Tomography (CT scan) or Magnetic Resonance Imaging (MRI). Patients with metastatic spread to the chest wall or involvement by direct invasion from intrathoracic neoplasms, patients opting out of surgery or unwillingness to participate in the study were excluded. Written informed consent was obtained from all patients. Approval of the study was taken from the Institutional Ethics Committee {IPGME&R Research Oversight Committee is registered with Central Drugs Standard Control Organisation (CDSCO), Government of India, in consonance with Rule 122D of the revised Drugs and Cosmetic Rules 1945- Registration No. ECR/35/Inst/WB/2013. It functions in accordance with revised Schedule Y and Indian Council of Medical Research (ICMR) guidelines}. Necessary clinical data about the enrolled patients was collected as per proforma.
All the patients have undergone surgical excision of the tumour masses. At the Department of Pathology, gross examination of the specimens was done followed by histopathological reporting. Special stain like Periodic Acid Schiff (PAS) was also done in some cases. The sections were subjected to IHC by peroxidase-antiperoxidase technique. Epithelial Membrane Antigen (EMA) {Monoclonal Mouse Antibody; clone: E29, positive control: skin, cytoplasmic and membrane positivity}, CD99 {Monoclonal Mouse Antibody; clone:12E7, positive control: oesophagus, membranous staining}, desmin {Monoclonal Mouse Antibody; clone: D33, positive control: skeletal and cardiac muscle cells, cytoplasmic staining}, S-100 {Polyclonal Rabbit Antibody; clone: 15E2E2, positive control: melanoma, cytoplasmic staining}, vimentin {Monoclonal Mouse Antibody; clone: V9, positive control: tonsil, cytoplasmic staining}, cytokeratin 7 (CK 7) {Monoclonal Mouse Antibody; clone: OVTL12/30, positive control: lung, cytoplasmic/membranous staining}, p53 {Monoclonal Mouse Antibody; clone: BP53-12-1, positive control: skin cancer, nuclear staining}, CD34 {Monoclonal Mouse Antibody; clone: QBEnd 10, positive control: bone marrow, membrane staining}, Bcl2 {Monoclonal Mouse Antibody; clone: 124, positive control: tonsil, cytoplasmic staining}, Leukocyte Common Antigen (LCA) (CD45) {Monoclonal Mouse Antibody; clone: 2B11+PD7/26, positive control: tonsil, membrane staining}, CD56 {Monoclonal Mouse Antibody; clone: 123C3, positive control: thyroid, membrane staining}, CD138 {Monoclonal Mouse Antibody; clone: MI15, positive control: tonsil, membranous staining}, were being used for the confirmation of the diagnosis. IHC were analysed qualitatively.
Statistical Analysis
Statistical forms were used to record the relevant demographic, clinical, laboratory data for each patient. Results were analysed using Microsoft excel 2016 and GraphPad InStat 3. Comparisons between two groups were performed using an independent sample t-test. A p-value <0.05 was considered statistically significant.
Results
Benign Tumours
Six (37.5%) of the 16 chest wall tumours were benign, five cases were primary rib tumours and one of soft tissue origin.
Fibrous Dysplasia
There were four cases of Fibrous Dysplasia (FD) of the rib. They constituted 25% of all the tumours and 66.7% of the benign tumours.
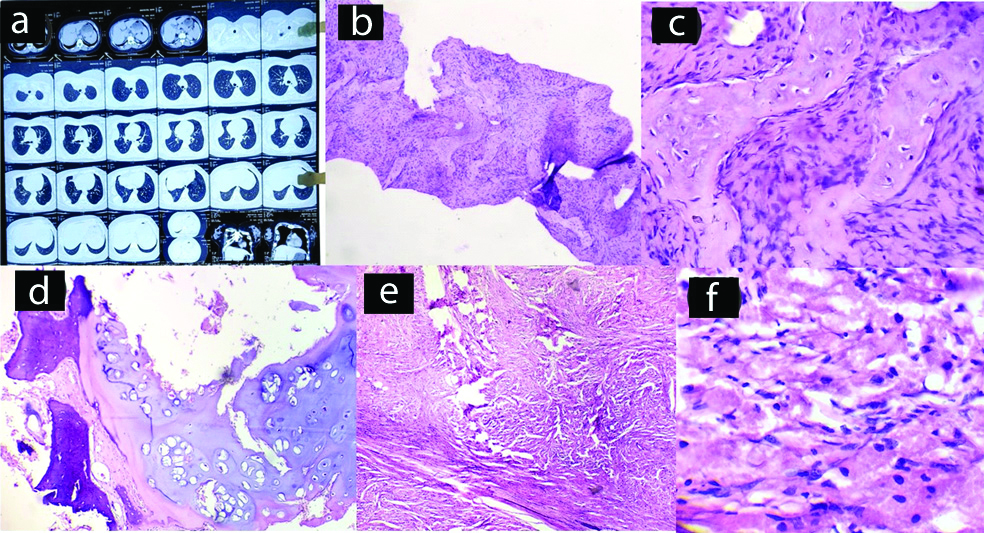
CXR and CT scan of the lesions showed lobulated, expansile lesion and thinning of the cortex [Table/Fig-1a]. The tumour masses were surgically excised. Grossly, it was a firm; well- defined solid, grey-white mass replacing the medullary cavity with areas of haemorrhage. Histology, showed disorganised irregular ‘Chinese character like’ spicules of woven bone separated by abundant fibrous stroma, bland spindle cells with no atypia of stromal cells [Table/Fig-1b,c]. The final diagnosis of FD was made.
Case of fibrous dysplasia. (a) CT scan of chest wall; (b) Section shows Chinese alphabet spicules of woven bone (x100, H&E); (c) Corresponding histological section (x400, H&E).
Case of enchondroma; (d) Section shows cartilage of variable cellularity (x100, H&E).
Case of granular cell tumour; (e) Section shows polygonal cells with eosinophilic granular cytoplasm, fibrous septae between the clusters (x100, H&E); (f) Corresponding histological section (x400, H&E).
Chondroma
A chondroma involved the right sixth rib of a 28-year-old woman. Radiologically, the chondroma appeared as a well-defined oval area. Excision of the tumour mass was done. Grossly, it measured 5×4×2 cm. Histological section showed variable cellularity of cartilage with binucleated chondrocytes, occasionally, but there was lack of nuclear pleomorphism and mitotic activity [Table/Fig-1d]. The final diagnosis was chondroma of the rib.
Granular Cell Tumour
A single case was reported, 47-year-old woman presented with a five-month history of a non-tender, firm mass at her right chest wall. The swelling was subcutaneous, nodular, measuring approximately 2.5×2 cm in size. There was no ulceration or pigmentation of the overlying skin. There was no palpable peripheral lymphadenopathy. Ultrasonographic (USG) evaluation of the swelling revealed a well-defined, heteroechoic lesion measuring 2.5×2×2 cm, present in the subcutaneous plane. Grossly, specimen (3.0×2.5×2 cm) consisted of pinkish, soft tissue and the cut surface showed a yellow homogeneous appearance. Microscopic examination revealed polygonal cells with eosinophilic granular cytoplasm, clusters of cells separated by fibrous septae [Table/Fig-1e,f]. Periodic Acid–Schiff (PAS) stained positive. IHC showed S100 positive and CK 7 negative. The diagnosis of granular cell tumour was confirmed.
[Table/Fig-2,3] shows frequency of benign and malignant tumours with age and sex distribution.
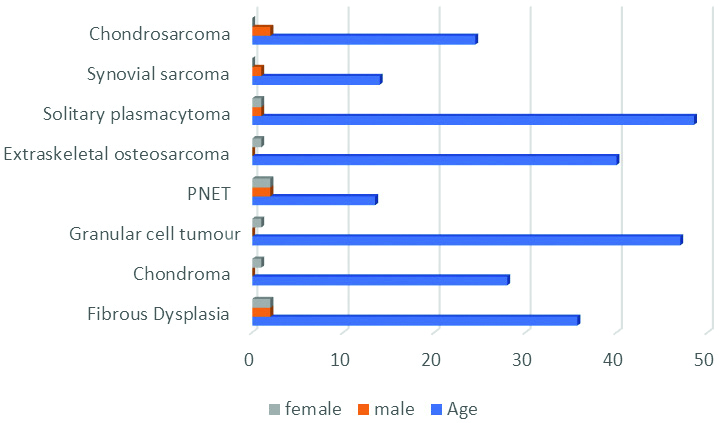
Frequency of primary chest wall tumours with age and sex distribution (N=16).
| Tumour type | Number of patients | Agerange (years) | Sex |
|---|
| Male | Female |
|---|
| Fibrous dysplasia | 4 | 25-45 (35.7) | 2 | 2 |
| Chondroma | 1 | 28 | 0 | 1 |
| Granular cell tumour | 1 | 47 | 0 | 1 |
| PNET | 4 | 10-18 (13.5) | 2 | 2 |
| Extraskeletal osteosarcoma | 1 | 40 | 0 | 1 |
| Solitary plasmacytoma | 2 | 45-52 (48.5) | 1 | 1 |
| Synovial sarcoma | 1 | 14 | 1 | 0 |
| Chondrosarcoma | 2 | 22-27 (24.5) | 2 | 0 |
Graphical representation of frequency of primary chest wall tumours with age and sex distribution (N=16).
Malignant Tumours
Among the 16 (62.5%) chest wall tumours, 10 were malignant. Four were rib tumours and six were chest wall tumours.
PNET (Peripheral Neuroectodermal Tumour)
PNET was encountered in four patients. This represents 25% of all the tumours and 40% of the malignant tumours. All of them originated from the soft tissue of chest wall.
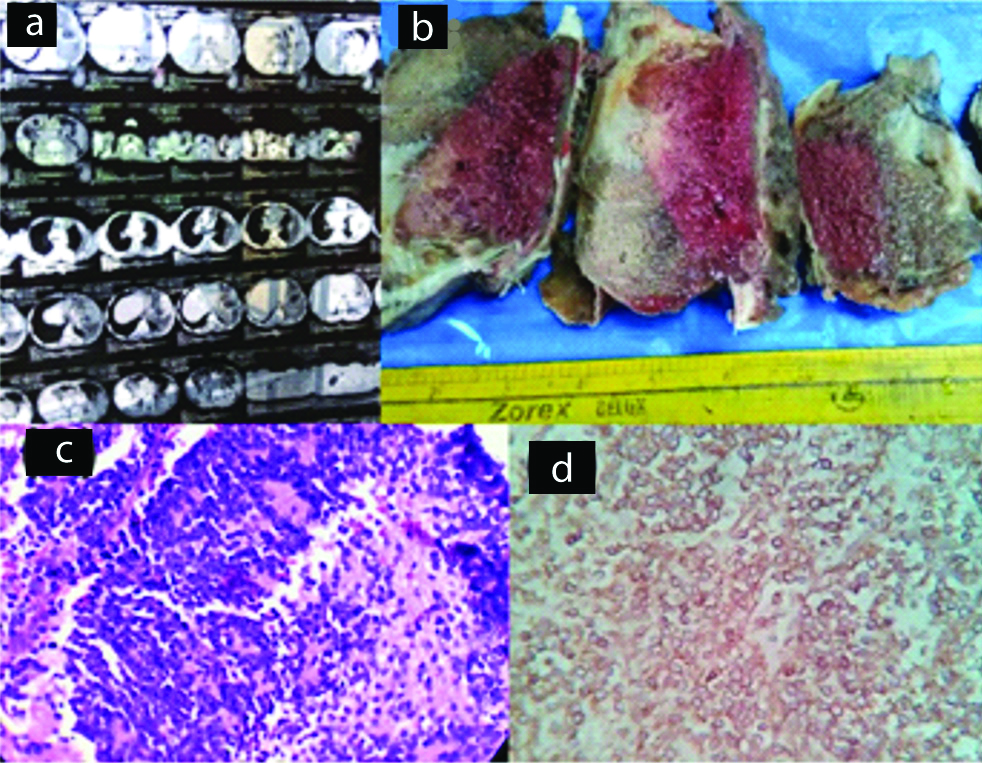
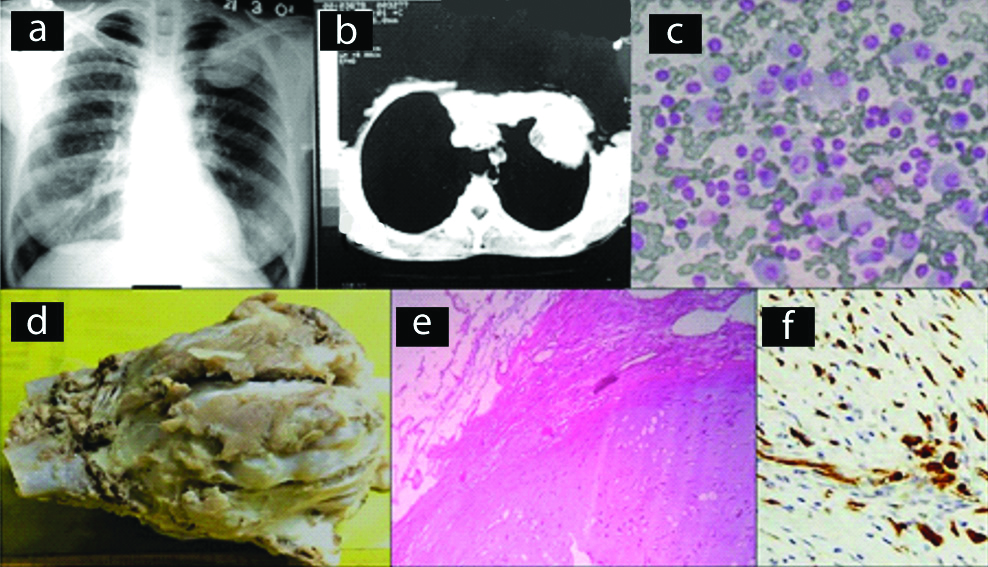
Contrast-Enhanced Computed Tomography (CECT) of the chest showed a heterogenous lobulated mass with heterogenous enhancement and hypodense areas suggestive of necrosis [Table/Fig-4a]. In some instances, MRI thorax identified the presence of a spindle-shaped mass on the anterolateral wall with an osteolytic lesion in the costal arch [Table/Fig-5a]. Grossly, the tumour lesions were firm, pale with variable sizes, and extensive, areas of haemorrhage and necrosis [Table/Fig-4b]. Microscopy of the tumour showed sheets of small round cells and presence of Homer-Wright rosettes [Table/Fig-4c,5b]. The microscopic appearance was not pathognomonic and the differential diagnosis included lymphoma. IHC showed strong positivity for CD99 [Table/Fig-4d,5c] and Vimentin but negative for CK 7, LCA (CD45). The diagnosis of PNET of chest wall was confirmed.
Case of PNET. (a) CT scan of chest wall showing soft tissue mass; (b) Gross specimen, cut section shows haemorrhage and necrosis; (c) Section shows sheets of small round cells (x400, H&E); (d) IHC staining for CD 99 showing positivity (x400).
Case of PNET. (a) MRI of chest wall showing soft tissue mass; (b) Section shows sheets of small round cells and rosettes formation (x400, H&E); (c) IHC staining for CD 99 showing positivity (x400).
Case of extraskeletal osteosarcoma; (d) Section shows vague like whorl formation (x100, H&E); (e) Section shows areas of calcification and osteoid formation (x100, H&E); (f) IHC staining for vimentin showing positivity (x400).
Extraskeletal Osteosarcoma
There was only a single case among the malignant tumours. A 40- year-old woman presented with right sided chest wall mass. CECT of the chest detected a 10 × 6 cm soft-tissue mass with irregular contours arising from right chest wall. Grossly, it was globular and measured 12 × 8 × 8 cm. The histological section showed a tumour mass composed of highly pleomorphic cells with hyperchromatic nuclei arranged in sheets [Table/Fig-5d]. There were extensive areas of necrosis and at some areas osteoblastic and chondroblastic differentiation [Table/Fig-5e] with areas of calcification and giant cells also noted. IHC showed positive staining for vimentin [Table/Fig-5f] and were negative for S-100, CD56 and CD34. The diagnosis of extraskeletal osteosarcoma was confirmed.
Solitary Plasmacytoma
There were two cases of solitary plasmacytoma. They constituted 12.5% of all the chest wall tumours and 20% of the malignant tumours.
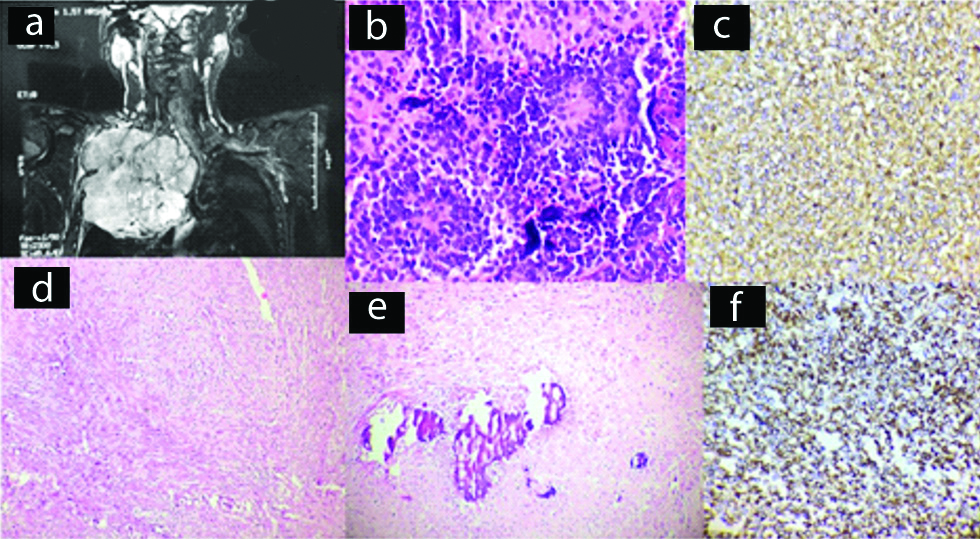
Radiographically, plasmacytoma revealed punched out lesions in the ribs [Table/Fig-6a,b]. Strands of bony spicules were noted in the outer cortex. Initially, FNAC was performed which showed plasmacytoid cells both mononucleate and multinucleate [Table/Fig-6c]. Then surgical excision was performed. Grossly, it was soft, pale and reddish. Microscopically, section revealed close packed cells with plasmacytoid features such as perinuclear halo, copious cytoplasm and eccentric nuclei. IHC showed positive staining for CD138. These cases were diagnosed as solitary plasmacytomas.
Case of solitary plasmacytoma. (a) Chest X-ray (AP view) revealed an osteolytic lesion on left side; (b) CT scan of chest wall showing osteolytic lesion with soft tissue mass; (c) FNAC smear shows plasmacytoid cells both mononucleate and multinucleate (x400, MGG).
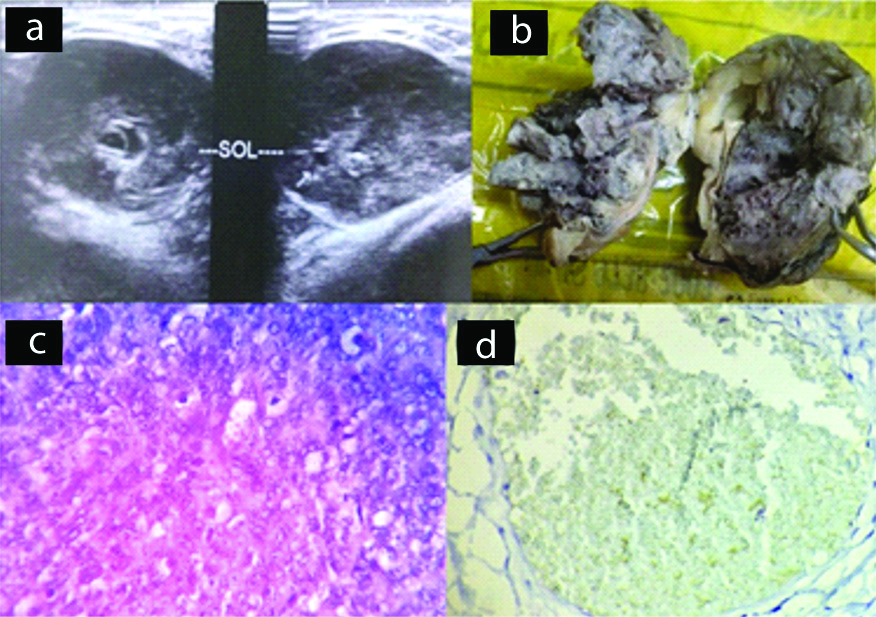
Case of chondrosarcoma; (d) Gross specimen, showing ribs with a soft tissue mass; (e) Section shows tumour mass infiltrating into adjacent soft tissue (x100, H&E); (f) IHC staining for S-100 showing positivity (x400).
Chondrosarcoma
Chondrosarcoma was found in two patients. Both of them originated from the ribs. These tumours constituted 20% of malignant tumours and 12.5% of all the tumours.
CECT of the chest revealed a large heterogeneous lobulated mass on the anterior chest wall which was osteolytic with some areas of calcification. The tumours ranged from 10 to 15 cm. Grossly, the tumours tend to be lobulated, smooth [Table/Fig-6d]. On cut section, the cartilaginous tumour showed areas of calcification. Histopathological examination revealed polygonal cells with centrally placed hyperchromatic nuclei with presence of nucleoli and large abundant vacuolated cytoplasm with splotchy calcification, enchondral ossification and entrapment of host lamellar bone [Table/Fig-6e]. Both were categorised as grade I. On IHC, S-100 [Table/Fig-6f] showed positivity and p53 was negative.
The diagnosis of chondrosarcoma was confirmed.
Synovial Sarcoma
A single case was reported, 14-year-old boy presented with anterior chest wall mass. He underwent an USG which was reported as oval hypoechoic mass lesion having smooth outline [Table/Fig-7a]. Further an USG guided FNAC was performed which was suggestive of small blue round cell tumour. Surgical resection of the mass was performed. The specimen measured 6x5 cm [Table/Fig-7b]. Histological sections showed a tumour mass composed of round oval cells arranged in solid sheets and lobules with monomorphic appearance [Table/Fig-7c]. The nucleus was vesicular, prominent nucleoli and scanty cytoplasm. The histological features suggested of small blue round cell tumour. On IHC, the tumour cells were positive for vimentin, CD99, Bcl2 [Table/Fig-7d] and S-100 protein. They were desmin, CD34, CK 7 and EMA negative. The diagnosis of Synovial Sarcoma (monophasic type) was confirmed.
Case of synovial sarcoma. (a) USG oval hypoechoic mass lesion having smooth outline; (b) Gross specimen; (c) Section shows tumour mass composed of round oval cells and mitotic figures (x400, H&E); (d) IHC staining for Bcl2 showing positivity (x400).
Discussion
Chest wall is the site of origin for both benign and malignant neoplasms of various types. They comprise for <5% of thoracic malignancies which may be primary or metastatic, with a malignancy rate of 50% [7] and either symptomatic or asymptomatic, with 20% found incidentally on chest radiograph [8]. For accurate diagnosis and treatment of the chest wall lesions, proper history and clinical examination, radiological investigations and histopathology along with IHC is important. The overall prognosis of the malignant chest wall tumours is poor and the patients here are all under follow-up.
The age range and duration of symptoms are not important diagnostic criterias for judging likely biological behaviour of lesion, since patients with benign and malignant tumours had overlapped age ranges considerably, similar to the ranges of duration of symptoms. Although six (60%) out of the 10 malignant cases and two (33.3%) out of six benign cases were painful; palpable swelling was present in 70% of the malignant tumours and 66.7% of the benign lesions [Table/Fig-2,3,8]. Associated features were pleural effusion, dyspnea. The clinical features which suggests that tumour is malignant are massive increase in size, adjacent structures being invaded and the metstasis, especially to the lungs. On the contrary, many of the malignant tumours in this study lacked the above mentioned features of malignancy.
Distribution of the primary chest wall tumours according to their symptoms and tumour location (N=16).
| Tumour type | Symptoms | Duration of symptoms | Tumour location |
|---|
| Swelling | Pain | Others | Bony structure (ribs) | Soft tissue |
|---|
| Fibrous dysplasia | 2 | 2 | Dyspnea (1) | 6 months-2 years | 4 | 0 |
| Chondroma | 1 | 0 | 0 | 1 year | 1 | 0 |
| Granular cell tumour | 1 | 0 | 0 | 5 months | 0 | 1 |
| PNET | 3 | 2 | Pleural effusion (1) | 6 months -1 year | 0 | 4 |
| Extraskeletal osteosarcoma | 1 | 1 | 0 | 4 months | 0 | 1 |
| Solitary plasmacytoma | 1 | 1 | 0 | 8 months-2 years | 2 | 0 |
| Synovial sarcoma | 1 | 1 | Dyspnea | 6 months | 0 | 1 |
| Chondrosarcoma | 1 | 1 | Pleural effusion (1) | 3 months-1 year | 2 | 0 |
In this study, there were some interesting differences from chest wall tumour characteristics commonly mentioned in other reviews. The rate of primary malignancy (62.5%) [Table/Fig-9] was significantly higher than normally reported. The sex distribution did not reveal any marked predilection for males (eight males, eight females) [Table/Fig-9].
Comparison between the benign and malignant chest wall tumours.
| Number of cases | Age | Sex | Tumour location |
|---|
| Male | Female | Bony structures (ribs) | Soft tissue |
|---|
| Benign | 6 (37.5%) | 25 to 48 years | 2 | 4 | 5 | 1 |
| Malignant | 10 (62.5%) | 10 to 52 years | 6 | 4 | 4 | 6 |
Primary malignant tumours outnumbered benign ones which correlated with the findings of Sabanathan S et al., [1]. But differs from Teitelbaum SL, Hochberg LA and Crastnopol P, Cavanaugh DG et al., [Table/Fig-10] [6,9,10].
Table showing the comparison with previous studies [1,6,9,10].
| Name of the study | Benign tumours | Malignant tumours |
|---|
| Sabanathan S et al., [1] | 49.1% | 50.9% |
| Teitelbaum SL [6] | 59.5% | 40.5% |
| Hochberg LA and Crastnopol P [9] | 52.4% | 47.6% |
| Cavanaugh DG et al., [10] | 73.3% | 26.7% |
| Present study | 37.5% | 62.5% |
FD is characterised by abnormal osteoblastic differentiation leading to replacement of normal marrow and cancellous bone by islands of immature woven bone with fibrous stroma [11,12]. FD was first described by Lichtenstein in 1938 and may involve single bone (monostotic) or multiple bones (polyostotic) [13]. Among the four cases, three were monostotic and one was polyostotic (other lesions were in skull involving sphenoid bone and was not associated with any syndrome). FD of ribs accounts for up to 30% of all benign chest wall tumours [14-16] and they typically present in the third or fourth decade of life. In present study, patients belonged to the age group of 25 to 45 years.
GNAS1 on chromosome 20, is responsible for the postzygotic-activating mutations of signal transducing G proteins. These types of mutation are associated with FD [17]. Here, the patients could not afford for the cytogenetic study. FD may be associated with McCune Albright syndrome (polyostotic FD, café-au-lait spots and endocrine dysfunction) and Mazabraud syndrome (polyostotic FD and soft-tissue myxomas), in its polystotic form [18]. One of the important differential diagnosis which is to be kept in mind clinically, radiographically and histopathologically is low grade osteosarcoma. Cytologically, it shows more atypical features, histologically, it exhibits high cellularity and mitotically more active than FD. The prognosis of patients with FD is good.
Of all the tumours, chondromas are relatively common, accounting for 10-25% incidence [19]. It is a benign hyaline cartilage neoplasm of medullary bone. Chondromas are uncommon in the flat bones such as ribs, scapula, pelvis, vertebrae or sternum. Occasionally, an enchondroma will recur many years later, and and recurrence is very rare as low grade chondrosarcoma [19].
Granular cell tumours are uncommon soft tissue neoplasm of nerve sheath origin, which are predominately benign. Granular cell tumour was first reported by Abrikossoff A by the name of granular cell myoblastoma [20]. They are commonly found in the age range of 10-50 yrs with female predilection as compared to males. Also, they have an incidence of 0.6% among all soft tissue tumours [21-24]. Here, in present case a female aged 47 years was affected.
Microscopically, they are composed of large polygonal tumour cells. These characteristic tumour cells exhibit relatively small nuclei and abundant granular cytoplasm. These granules are characteristically PAS positive and diastase-resistant and immunoreactive to S100 and CD68. Differential diagnosis includes rhabdomyoma, hibernoma, oncocytoma. This case was diagnosed as benign granular cell tumour as they lack the features of necrosis, vesicular nuclei with prominent nucleoli and increased mitotic activity (more than 2/10 HPF) [25]. Malignant granular cell tumours comprise less than 1% of all granular cell tumours and the most common metastatic sites of malignant granular cell tumour are the lymph nodes, followed by the lungs [26].
PNET, commonest pediatric chest wall malignant neoplasm and comprises incidence of 6%-11% of all PNETs. Other name for PNET is Askin- Rosai tumour and it originates from embryonic cells of the neural crest [27-31] and may affect the ribs, sternum, scapula, clavicle and soft tissues of the chest wall [32]. PNET of the chest wall belongs to the Ewing’s sarcoma family due to their identical chromosome translocation t (11; 22) (q24; q12) giving rise to the EWS/FLI-1 fusion gene [33] and phenotypic appearance. The incidence peak is between 15 and 25 years so as in present four cases. Distant metastases occur in lungs, bones, bone marrow, liver and brain [29]. Other small round cell tumour such as lymphoma comes as differential diagnosis. The overall survival rate is 93% and the 10-year probability of survival is estimated at approximately 90% [32].
Osteosarcoma is a high grade tumour which typically develops in long bones of adolescents and young adults [34]. Extraskeletal osteosarcoma is rarest form (~5%) with very aggressive behaviour, located in the limbs of adult patients (older than 40 years) [35]. Here, patient was a female aged 40 years. Extraskeletal osteosarcoma which presents in thoracic locations are very rare and exhibits very poor prognosis. commonest site for metastasis was lung (55%) followed by kidney, brain or adrenal glands [35].
Tumour of plasma cells can manifest as Multiple Myeloma and Solitary Plasmacytoma. Plasmacytoma is further classified into 2 groups-Osseous (solitary plasmacytoma of bone) and Non-osseous {Extra Medullary Plasmacytoma (EMP)} [36]. This predominantly occurs in patients over 60 years of age and more common in men than in women [37]. In present study, a 45-year-old female and 52-year-old male were affected. An 80% of EMP occurs in head and neck, especially the nasopharynx and paranasal sinuses. Rare cases have been described in the skull base, larynx, hypopharynx, chest wall. The diagnostic criteria for solitary plasmacytoma includes: biopsy proven solitary lesion of bone or soft tissue consisting of clonal plasma cells, normal random bone marrow biopsy with no evidence of clonal plasma cells, normal skeletal survey and MRI or CT (except for the primary solitary lesion), absence of end organ damage such as hypercalcaemia, renal insufficiency, anaemia, bone lesions [19]. Both of the patients of present study met these criteria. The potential for malignant systemic progression is higher for solitary plasmacytoma of bone than extramedullary plasmacytoma [38]. The prognosis for extramedullary plasmacytoma with multiple myeloma is poor and most patients die within two years of diagnosis. The 3-year survival rate is only about 10% [19].
Chondrosarcomas are malignant tumours that produce chondroid matrix. Primary chondrosarcomas arise de novo. Secondary chondrosarcomas occur in pre-existing benign cartilaginous neoplasms [39]. They most commonly affect flat bones such as pelvic and shoulder girdle, however approximately 15% present in the chest wall with annual incidence of 0.5 per million [40]. They tend to occur in a slightly younger patient population, associated with genetic aberrations involving chromosome 12q13-15 and 9p21 [41]. Grading is based on histological features, i.e., the matrix composition, presence of nuclear atypia and mitotic rate. Grade I as of best prognostic factor and II, III are of worse prognosis. The two cases reported here, belonged to Grade I. The five-year survival is 89% for patients with grade 1, the combined group of patients with grade 2 and 3 have a five-year survival of 53% [41]. The most common sites of metastasis are the lungs, soft tissues, lymph nodes, bone and the brain [40].
Primary synovial sarcomas of the chest wall are extremely rare [42,43] and may arise within the mediastinum, heart, lung, pleura, or chest wall. A specific balanced reciprocal translocation, t(X;18) (p11.2; q11.2), is found in more than 90% of all synovial sarcomas. The translocation involves the SYT gene on chromosome 18 and either the SSX1 or SSX2 gene on the X chromosome [43]. The patient could not afford for the cytogenetic study. Synovial sarcomas are broadly classified into: (I) biphasic type; (II) monophasic fibrous type, and this case belonged to monophasic type. IHC stains strong positivity for vimentin, bcl-2, and CD99 in the spindle cells. Bone, liver, skin, CNS are the sites for the metastasis [44]. Prognosis is related to the disease stage and is usually poor.
Limitation(s)
The probable reason for differences between present study findings and those of others is that chest wall lesions occur relatively rarely, making consistent patterns difficult to develop. This study was done on small number of patients and for a shorter time period. Additionally, present study sample is derived from a general population rather than from a select referral population.
Conclusion(s)
Chest wall neoplasms are group of heterogeneous lesions with varied pathology. Any tumour arising from chest wall should be inspected properly to ascertain the origin of the tumour for a definitive diagnosis. Clinical and imaging correlation along with histopathology and IHC aids in proper diagnosis.