The HIV is a causative agent of dreadful disease known as Acquired Immunodeficiency Syndrome (AIDS). Since contemporary medicine failed to deliver a cure for it has marked HIV one the feared and prevalent virus of current times. Today millions of human beings are infected with HIV with various modes of transmission. It is estimated that 37.9 million people are living with HIV infection as per 2018 estimates [1].
Genetic composition of HIV consists of double identical single stranded RNA molecules and these are enclosed within a core of virus particle [2]. HIV-1 preferentially infects T cells with high levels of CD4 and subsets of T cells that express CCR5, particularly memory T cells which is necessary for cell fusion and viral entry into the immune cell. Though binding to CD4 receptor is not itself enough, it requires partaking of co-receptor molecule which has been recognised as CCR5 for HIV-1 virus [3]. Deletion of 32-base pair in the CCR5 gene, such as homozygous genotype acts as a nonfunctional receptor, which confirms natural resistance to HIV-1 infection [4]. Important fact that heterozygous genotype (CCR5 Δ32/WT) may help to a slower progression to AIDS compared with CCR5-WT/WT genotype [5].
Bone marrow or peripheral blood or umbilical cord blood alone or together used as source of multipotent haematopoietic stem cells routinely used nowadays for Haematopoietic Stem Cell Transplantation (HSCT) is the transplantation. In this HSCT procedure, using a donor with a homozygous mutation of co-receptor CCR5 (CCR5 Δ32/Δ32) can lead to HIV-1 remission which is efficient, less aggressive and less toxic approach [6]. The low prevalence of the CCR5 Δ32/Δ32 genotype and stringent HLA-matching criteria in case of Bone Marrow HSCT will make it very difficult to identify suitable related or unrelated adult volunteer donors. Nevertheless, the higher tolerance to HLA mismatch in Umbilical Cord Blood Transplantation (UCBT) and their immediate availability in umbilical cord blood inventories may make it the ideal cell source [7], as a drug-free durable HIV-1 suppression, which is, therefore, an urgent global priority.
The number of HIV patients in India are at alarming rate and therefore, this study was performed to work on CCR mutation in Indian umbilical cord blood samples which will be helpful for the future potential use of HSCT for HIV-1 cure.
Materials and Methods
This cross-sectional study for CCR5 genotyping was carried out on randomly selected Umbilical cord blood samples over a period of six months (from June 2019 to November 2019) at Regrow Biosciences Private Limited, Lonavala, Pune, Maharashtra, India. All the umbilical cord blood samples were obtained from designated Maternity Hospital with informed consent from donor with Institutional Ethical Committee for Stem Cell Research approval for this study (Approval No.: REG/IC-SCR/20/001). Ex-utero collection of Cord blood was done within 5 minutes after clamping the cord. The sample which was found negative for infectious diseases viz., HIV, HBsAg, HCV, CMV, HTLV and Syphilis were used for this study. One ml of whole cord blood or 0.5 mL of buffy coat (leucocyte rich processed cord blood) were used for the isolation of DNA. DNA samples were extracted from 800 Umbilical cord blood samples for CCR5 genotyping.
DNA Isolation and Quantification
DNA isolation was carried out using commercially available Genomic DNA extraction Kit (FavorPrep, Make: Favorgen). Isolated genomic DNA quantified using UV based fluorometer (Cubit 4, Make: Thermofisher Scientific). An average of 10 μg of total DNA from whole blood, and up to 50 μg of DNA from buffy coat was isolated. This genomic DNA was used for the CCR5 genotyping.
Genotyping of CCR5 Delta 32 Mutation
Initially, CCR5 genotyping of umbilical cord blood samples were screened using Automated Thermal Cycler (Veriti Dx 96 well, Make: Thermo Fisher Scientific). The samples positive for CCR5 mutation were confirmed by capillary electrophoresis methods.
In PCR based method, for CCR5 genotyping two specially designed, forward and reverse primers encompassing the 32 bp deletion were used.
The forward primer: 5’ CTC CCA GGA ATC ATC TTT ACC AGA TC-3’
The reverse primer: 5’ CTT CTC ATT TCG ACA CCG AAG CAG AG-3’,
The deletion in CCR5 was assayed by means of the PCR with above primers. PCR was carried out with 20 pmol of each primer, 10 mM mixed dNTPs, 5 mM MgCl2, and 1 unit of Taq polymerase in a final volume of 25 μL. Cycle conditions were: Initialisation step at 95°C for 10 minutes, denaturation step at 95°C for 30 seconds and Annealing step at 56°C for 30 seconds followed by final elongation step at 72°C for 10 minutes for 35 cycles. A sample of the reaction product (10 μL) was run on 2% Agarose gels with a 100 BP ladder and visualised by ethidium bromide staining using gel doc instrument.
The products from deletion-containing samples generate either a single band of 172 base pair from the homozygous deletions or two bands of 204 and 172 base pair from the heterozygous samples or the wild type allele resulted in 204-bp product.
The samples show positive for CCR5 mutation, were confirmed using DNA capillary electrophoresis system. These samples were outsourced to Phalanx biotech Taiwan.
Statistical Analysis
Statistical analysis was calculated using Fisher’s formula. Confidence level was determined at 95% with Confidence interval of 3.46. This data were derived from Indian population, where calculated sample size was 800.
Results
The current study sample results are summarised in [Table/Fig-1]. The 204-bp band represented the wild-type alleles and the 172-bp band represented the CCR5Δ32/WT allele (heterozygous genotype). Totally, 21 mutant alleles (21 heterozygotes and no homozygote) were detected among all the samples. The prevalence of CCR5Δ32/WT allele was 2.62%.
CCR5 Genotype forms and frequency.
| Sr. no. | Genotype form | Number | % |
|---|
| 1 | Total samples tested | 800 | 100 |
| 2 | Normal homozygote CCR5Wild/ Wild | 779 | 97.37 |
| 3 | HeterozygoteCCR5Wild/Δ32 | 21 | 2.62 |
| 4 | Mutant homozygoteCCR5Δ32/Δ32 | 0 | 0 |
The wild-type CCR5 allele generated at 204-bp product whereas the CCR5Δ32/WT allele (heterozygous) double band generated at 172-bp product, i.e., 32 bp shorter than the wild type [Table/Fig-2].
CCR5 Genotype forms and frequency.
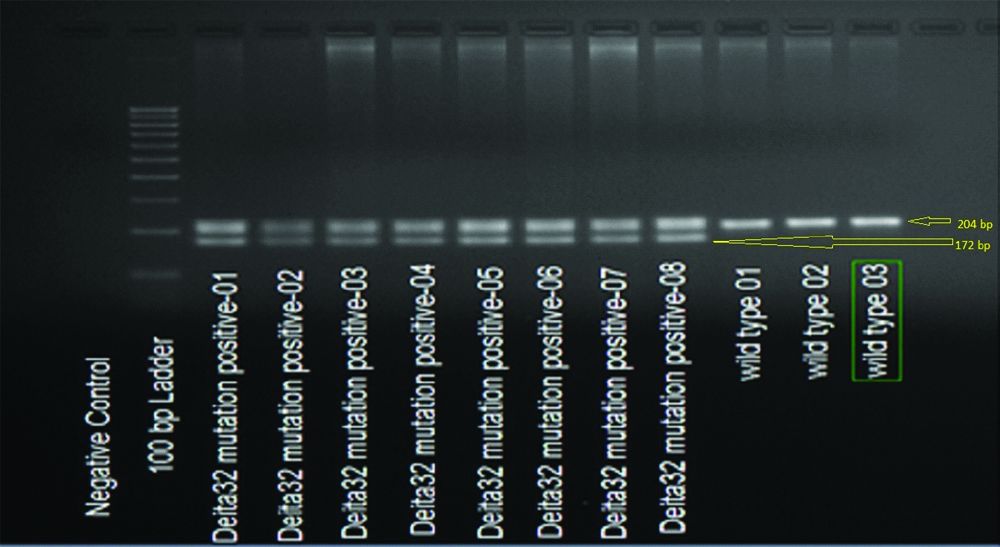
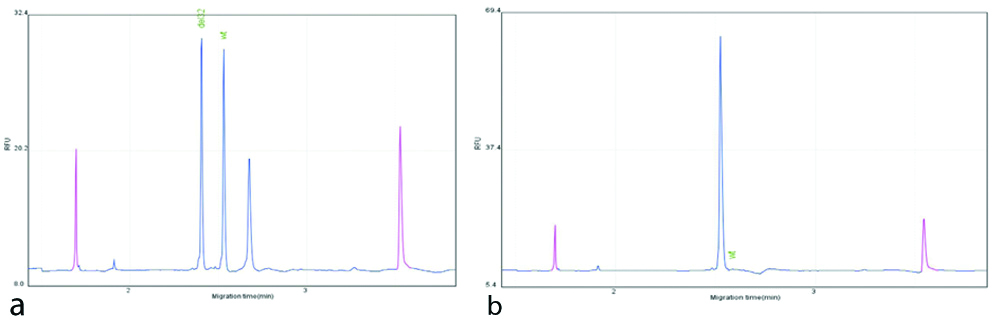
The samples with mutant alleles, showed the confirmation when tested using DNA capillary electrophoresis [Table/Fig-3a,b].
a: PCR analysis of CCR5 genotype: Total DNA was extracted from Umibilical cord blood and used for PCR amplification. Contemplated sizes for functional CCR5 are 204-bp and for wt/Δ32-functional heterozygous are 172 bp; b: GeneScan analysis of a sample heterozygous for the CCR5Δ32 allele. Fluorescence-labelled PCR products (blue peaks) were separated by capillary electrophoresis with a size standard (red peaks). (a) CCR5Δ32/WT (b) CCR5
WT/WT
Discussion
Over 40 years after the onset of the HIV pandemic, the HIV infection and AIDS is still a biggest challenge for humankind, even after the advent of highly active Antiretroviral treatment or by gene therapy. However, in last decade, one case of HIV-1 positive patient was functionally cured by HSCT with CCR5Δ32 homozygous units [8]. As per reports, even after more than a decade of post stem transplant in Timothy Ray Brown, also known as “The Berlin Patient,” virus did not reappear after stopping the treatment. The blood samples of this patient when tested repeatedly showed no detectable HIV as indicated by analysis of viral RNA as well as cellular proviral DNA. Apart from this T cells (CD4+) count shown to be at normal range. Another case was reported in 2019, a person (“London patient”) who has achieved HIV remission for at least 18 months [9].
The clinical applications of CCR5 32 bp deleted mutant cells is very restricted owing to the infrequent availability of donors with homozygous CCR5 Δ32 genotype [10]. This gene rate of occurrence in homozygous individuals is approximately 1% whereas heterozygous genotype of the CCR5-Δ32 allele in the European population is about 10% [11]. In this study, the selected 800 Cod Blood Units (CBU) samples for screening of CCR5 genotyping using PCR based method, where out of 800 samples, 21 samples exhibited CCR5 Δ32/WT genotype (heterozygous) which represent 2.62% and no other sample showed CCR5 Δ32/ Δ32 (Homozygous) genotype.
In current study, confirmation of the heterozygous genotype was done using capillary DNA electrophoresis system. In the DNA diagnostic field, this method is considered as an important diagnostic tool for its wide range of analytical applications [12].
The CCR5 is up-regulated by pro-inflammatory cytokines and is seen commonly in memory T cells, macrophages and immature dendritic cells. CCR5 gene is present on human chromosome 3 and the important part played by the CCR5 protein in human body is to interact with chemokines that signal white blood cell invasion into inflamed or infected tissues. CCR5 receptor is also very essential in HIV-1 entry into the cell [13]. A 32 base pair deletion in CCR5 gene as a result of mutation, leads to formation of a truncated protein that is not expressed on the cell surface, due to which HIV-1 virus could not recognise the host cells. The deletion confers resistance to HIV-1 infection in homozygous population and slows the progression of AIDS in HIV infected individuals of heterozygous population [14].
Other diseases that have been linked to the CCR5 and the Delta 32 mutation may include increased susceptibility to asthma [15]. Also, CCR5 Δ32 mutation accelerates and improves the treatment process of an Hepatitis B Virus (HBV) infection. The individuals who have a heterogenic form of CCR5-Δ32 are more likely to recover from HBV infection [16]. Moreover, CCR5 Δ32 heterozygous mutation can block the progress of cerebral malaria from human malaria parasites of the genus Plasmodium [17]. Additionally, CCR5 Δ32 heterozygous mutation can slow the progression of immune diseases such as Multiple Sclerosis [18].
The CCR5Δ32 mutation is an immunomodulatory response. Hence, Graft-versus-Host Disease (GvHD) is less common among the recipients whose donors are CCR5Δ32 homozygous compared to patients with heterozygous or wild-type donors [19].
As per literature, about 7,000 years ago the CCR5-del32 mutation may have originated in northeastern Europe [20]. Hence, its occurrence has now reached a reasonably higher side in Europeans, e.g., 16.3% in Finns and 15.8% in Moravians, it is not seen amongst African populations, and is only at low levels in the Asian [11]. Hence, we need to screen larger population for significant results in terms of homozygous CCR5 Δ32/ Δ32 genotype.
The main goal of this study was to develop an inventory of CCR5 Δ32/Δ32 HLA typed CBUs ready for UCBT and to explore their biological characteristics of interest. Also to facilitate the future development of clinical trials using allogenic CBUs for HIV -I treatments.
Limitation(s)
The limitation of current study was dearth of information due to deficiency of similar Indian referenced data on CCR5 genotype which resulted in comparison with international data. More CCR5 genotyping data on Indian population is required to draw more significant conclusion.
Conclusion(s)
The present study indicates that prevalence of heterozygous CCR5 delta 32 mutation is rare in Indian population. Hence, to obtain a sufficient number of CBUs with CCR5 Δ32/Δ32 genotype, large scale screening of said genotype is required. In addition, this study may be significantly helpful to encourage Indian cord blood bank depositories to analyse their Cord Blood inventory for CCR5 genotyping, which will be helpful to prepare CCR5-Δ32 cryopreserved cord blood units database.
Equally, for HIV-1 patients who are suffering from haematological malignancies and are in need of Haematopoietic stem cell transplantation, efforts should be intensified to identify CBU with CCR5 Δ32/Δ32 genotype. Physicians have to be keen to ensure success of the transplant at the same time to minimise the risk of GvHD. Under such circumstances, CCR5-negative CBU should remain second option for the treatment. Current study suggests that this PCR based method developed will be helpful for development of CCR5Δ32 CBU inventories. Further, this test may be included in the typing policy of public and private CBBs to improve the offer of an appropriate donor to eligible HIV-1 patients.