Candida krusei Infection in an Acute Lymphocytic Leukaemia Patient: A Case Report
Aroop Mohanty1, Suneeta Meena2, Uttam Kumar Nath3, Sudeep Vaniyath4, Neelam Kaistha5
1 Assistant Professor, Department of Microbiology, AIIMS, Rishikesh, Uttarakhand, India.
2 Assistant Professor, Department of Laboratory Medicine, AIIMS, New Delhi, India.
3 Additional Professor, Department of Medical Oncology and Haematology, AIIMS, Rishikesh, Uttarakhand, India.
4 Senior Resident, Department of Medical Oncology and Haematology, AIIMS, Rishikesh, Uttarakhand, India.
5 Professor, Department of Microbiology, AIIMS, Rishikesh, Uttarakhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aroop Mohanty, Assistant Professor, Department of Microbiology, AIIMS Rishikesh, Veerbhadra Marg, Rishikesh-249203, Uttarakhand, India.
E-mail: aroopmohanty7785@yahoo.com
Although Candida albicans remains the predominant species causing Blood Stream Infection (BSI), recent studies have demonstrated an emergence of Non-albicans Candida spp (NAC), such as C. glabrata, C. papapsilosis and C. krusei. Candida krusei are yeast-like microorganisms which may colonise the human skin, respiratory or gastrointestinal tract but can cause lethal infections especially in immunocompromised patients. Hereby, author’s report a case of 10-year-old male with T-cell Acute Lymphocytic Leukaemia (ALL) who reported with fever and non-productive cough caused by Candida krusei. Direct gram stain from blood culture and germ tube test was performed. Further, the isolate was initially misidentified by Matrix Assisted Laser Desorption/Ionisation Time of Flight Mass Spectrometry (MALDI-TOF MS) as Trichosporon ovoides which was later identified by molecular sequencing as Candida krusei.
Candida species, Fungemia, Haematological malignancy
Case Report
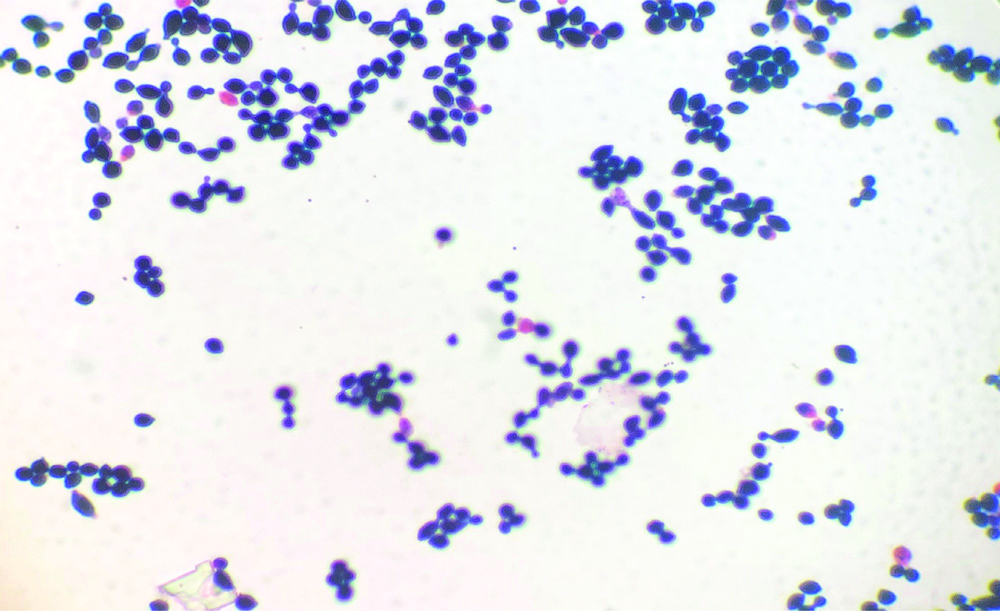
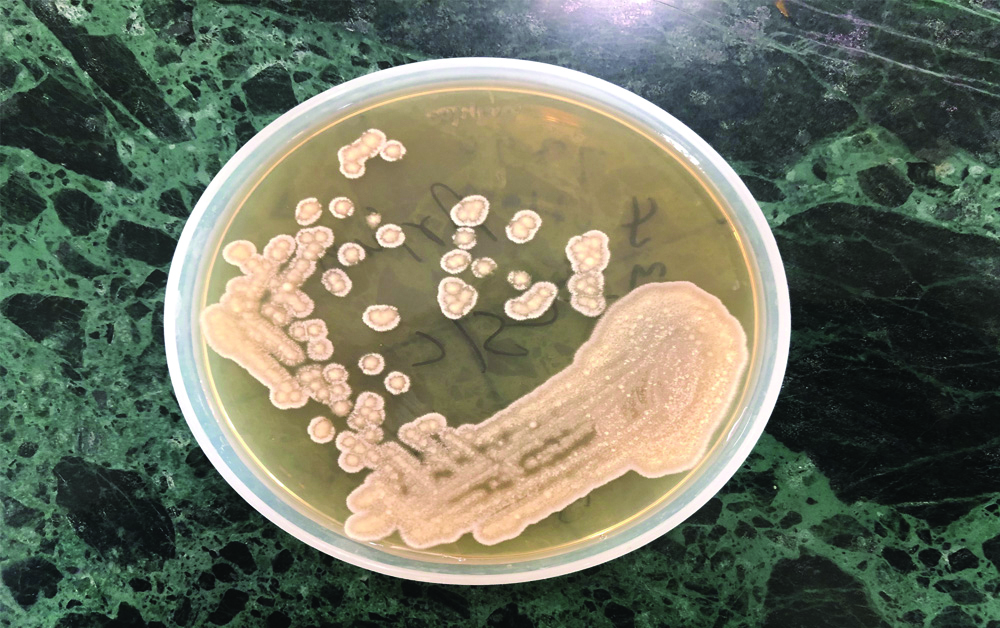
A 10-year-old male with T-cell ALL, who had received high-dose consolidation chemotherapy (HR3 block of ALL IC-BFM 2009 protocol) [1], was admitted on day 11 of chemotherapy cycle with fever and non-productive cough. An informed consent was obtained from the patient. On physical examination, the patient was febrile (100.4°F/38°C) with a heart rate of 120 beats/min, respiratory rate of 24/min, and blood pressure of 70/40 mmHg. His Total Leukocyte Count (TLC) was 480/μL and Platelet Count was 11000/μL. There was no family history for any such similar illness. Blood culture sample was sent from the indwelling Peripherally Inserted Central Catheter (PICC) and empiric intravenous antibiotics were started (Piperacillin-Tazobactam, Teicoplanin and Amikacin) for febrile neutropenia. His cough and fever worsened and TLC dropped from 250 μL/on day 12 to 103/μL on day 13. In view of the substantial decline of the white blood cells, the antibiotic regimen was escalated to Meropenem (1 g IV q8hr) and antifungal therapy was also started with liposomal Amphotericin B (3 mg/kg/day). His blood culture beeped positive on day 14 after which the PICC line was removed. Direct gram stain from the blood culture bottle revealed gram-variable budding yeast cells [Table/Fig-1]. Within 24 hours of subculture, white creamy colonies were obtained on blood agar and Sabouraud’s Dextrose Agar (SDA) (Hi Media India) [Table/Fig-2]. Germ tube test was performed using C. albicans ATCC 90028 as control and demonstrated negative results for germ tube production. It was further identified as Trichosporon ovoides by MALDI-TOF MS (Bruker Daltonics, Bremen, Germany) with a Confidence Interval (CI) of 1.41 by direct formic acid extraction. Antifungal susceptibility was performed on VITEK 2 (Biomerieux, Craponne, France). It was found resistant to Fluconazole (MIC ≥64 μg/mL), Flucytosine (≥64 μg/mL), Amphotericin-B (≥1 μg/mL) and was found susceptible to Voriconazole (MIC ≤0.25 μg/mL). Antifungal therapy was changed from Amphotericin B to Voriconazole (6 mg/kg/day q12h on day 1, followed by 4 mg/kg q12h for 7 days). He became afebrile on Day +17, and was discharged in stable condition.
Gram stain showing gram-variable budding yeast cells.
White, creamy pasty colonies seen on sabouraud’s dextrose agar.
However, in order to confirm the identity of the isolate, Internal Transcribed Spacer (ITS) regions (ITS1-5.8S-ITS2) were amplified using ITS1 (5′-TCCGTAGGT GAACCTGCGG-3) and ITS4 (5′-TCCTCCGCTT ATTGATATGC-3′) by National Culture Collection of Pathogenic Fungi (NCCPF) PGI, Chandigarh, India. Comparison of the nucleotide sequence of the isolate obtained with the Gene Bank database using the Basic Local Alignment Search Tool (BLAST) algorithm yielded 100% homology with the ex-type strain of C. krusei.
The patient came for follow-up two weeks later, when a repeat blood culture was taken which came out to be sterile and proved that he responded to Voriconazole therapy.
Discussion
In United States, Candida spp. are considered as the third leading pathogen which is responsible for BSI. In India Candida spp. is the fourth leading cause of BSI in hospitalised patients, with an associated mortality of 40-50% [2]. Although candidiemia is not as prevalent as bacteraemia, it is associated with high morbidity and mortality in both immunocompromised and immunocompetent critically ill patients [3]. A worldwide systematic review of Candida BSI prevalence found that Candida krusei constantly made up 1%-4% of infections regardless of geographic location, whereas two studies reported proportions of 8.5% and 10.6% in Finland and France respectively [4].
Risk of Candida infection is high when it is associated with risk factors like febrile neutropenia, chemotherapy, central venous catheters, steroid therapy, ventillators, urinary catheterisation, total parenteral nutrition, HIV infection, prolonged hospital stay, haematological and gastric malignancy, prior azole use, prior monoclonal antibody use and prior β-lactam/β-lactamase inhibitor use [5,6].
C. krusei has emerged as a notable pathogen, especially in immunocompromised patient groups in nosocomial settings. Widespread use of Triazole in these patients has contributed to a selective increase in C. krusei infection. In haematological malignancies, patient is given a cocktail of antibacterial, antifungal agents for prophylaxis. In a study conducted in Lebanon, increased consumption of antifungals by haematology department was observed. They also noticed that NAC spp. constituted 59% of all BSI with most common being Candida krusei [7]. In another study conducted by Abbas J et al., 49 patients (86%) with prior antifungal exposure were found to have colonisation with Candida krusei [8]. Presence of central venous catheters allows formation of biofilm by C. albicans followed by C. krusei which are known to be a very high biofilm producer with predilection to mucosal surfaces and respiratory tract compared to other NAC. This biofilm confers higher resistance to antifungals. Quantification of biofilm can be done by XTT assay (tetrazolium salt reduction) assay where there is reduction of XTT to formazan.
Recently, there is a shift from C. albicans to NAC spp. as the predominant infecting species in patients with cancer who had candidiemia. Among the factors that have been proposed to explain this shift in the distribution of the Candida spp. responsible for candidiemia is the prevalent use of Fluconazole and Miconazole as a prophylactic antifungal agent, especially in patients with haematologic malignancies and recipients of bone marrow transplantation [9]. Fluconazole resistance by C. krusei is 96.6% through various mechanisms like increased ABC transporter expression, low affinity of Fluconazole to ergosterol-11, aneuploidy [10].
Regardless of the types of antifungal therapy used, the inferior response in patients with haematologic malignancies may cause by the severity of the infection and the status of the underlying disease. The other reason which is immunity against NAC is poorly understood. Candida in blood may disseminate into brain by crossing blood brain barrier and phagocytosed by microglial cells. In Candida krusei, yeast transform into pseudohyphae in microglial cells, and escape immunity (nitric oxide stress of phagocytes) by vomocytosis [11]. This morphological transformation is not observed in other NAC spp.
Patients with haematologic malignancies are found to be admitted to the ICU more frequently, were neutropenic during the course of their candidiemia, and were associated with poor outcome and high mortality [12]. In addition, the overall response was found to be better in patients who did not receive prior antifungal prophylaxis regardless of the treatment modality [9].
The use of MALDI-TOF MS has revolutionised the diagnosis of fungal infections as it has enabled us to identify rare and non-sporulating types of fungi. But in this case, it misidentified a simple yeast like colony i.e., Candida krusei as Trichosporon ovoides. This highlights the fact that MALDI-TOF MS cannot be taken as the gold standard for identification of fungal isolates and proves that molecular sequencing should be done, if possible to confirm the MALDI-TOF MS findings. In this case, the diagnosis could have missed as we relied heavily on the MALDI-TOF MS finding and started the patient on voriconazole thinking of Trichosporon ovoides as the causative fungi. Correct identification is of utmost important as antifungal susceptibilities are different for different fungi and it helps to start the right treatment for the right patient.
Conclusion(s)
This case highlights the need for constant lookout for Non-albicans candidiemia especially in immunocompromised patients. In this case, the presence of central line along with haematological malignancy was purported to cause candidiemia. It was also concurred from this case that MALDI-TOF MS cannot be relied upon totally for the confirmation of such yeast like species and molecular sequencing should always be performed before the initation of antifungal therapy especially in such group of patients.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 11, 2020
Manual Googling: Apr 21, 2020
iThenticate Software: Apr 27, 2020 (11%)
[1]. Chemotherapy regimens for Pediatric ALL. Second Meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines Geneva, 29 September to 3 October 2008. https://www.who.int/selection_medicines/committees/subcommittee/2/cytotoxic_review.pdf [Google Scholar]
[2]. Duggal SD, Jena PP, Gur R, Kumar A, Rongpharpi SR, Pandey M, Recurring events of Candida krusei septicemia: First report from an ICUJournal of Mycology 2015 2015:72142410.1155/2015/721424 [Google Scholar] [CrossRef]
[3]. Deorukhkar SC, Saini S, Echinocandin susceptibility profile of fluconazole resistant Candida species isolated from blood stream infectionsInfectious Disorders- Drug Targets 2016 16(1):63-68.10.2174/187152651666615120915544726648186 [Google Scholar] [CrossRef] [PubMed]
[4]. Falagas ME, Roussos N, Vardakas KZ, Relative frequency of albicans and the various non-albicansCandida spp among candidiemia isolates from inpatients in various parts of the world: A systematic reviewInt J Infect Dis 2010 14(11):e954-66.10.1016/j.ijid.2010.04.00620797887 [Google Scholar] [CrossRef] [PubMed]
[5]. Kronen R, Hsueh K, Lin C, Powderly WG, Spec A, Creation and assessment of a clinical predictive calculator and mortality associated with Candida krusei bloodstream infectionsIn Open Forum Infectious Diseases 2018 5(2):ofx25310.1093/ofid/ofx25329450209 [Google Scholar] [CrossRef] [PubMed]
[6]. Giri S, Kindo AJ, A review of Candida species causing blood stream infectionIndian J Med Microbiol 2012 30(3):270-78.10.4103/0255-0857.9948422885191 [Google Scholar] [CrossRef] [PubMed]
[7]. Awad L, Tamim H, Abdallah D, Salameh M, Mugharbil A, Jisr T, Correlation between antifungal consumption and the distribution of Candida species in different hospital departments of a Lebanese medical CentreBMC Infectious Diseases 2018 18(1):58910.1186/s12879-018-3512-z30453891 [Google Scholar] [CrossRef] [PubMed]
[8]. Abbas J, Bodey GP, Hanna HA, Mardani M, Girgawy E, Abi-Said D, Candida krusei fungemia: An escalating serious infection in immunocompromised patientsArchives of Internal Medicine 2000 160(17):2659-64.10.1001/archinte.160.17.265910999981 [Google Scholar] [CrossRef] [PubMed]
[9]. Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I, The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidiemia in hematologic malignancyCancer: Interdisciplinary International Journal of the American Cancer Society 2008 112(11):2493-99.10.1002/cncr.2346618412153 [Google Scholar] [CrossRef] [PubMed]
[10]. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD, Azole antifungal resistance in Candida albicans and emerging Non-albicans Candida speciesFront Microbiol 2017 7:217310.3389/fmicb.2016.0217328127295 [Google Scholar] [CrossRef] [PubMed]
[11]. Sanches MD, Mimura LA, Oliveira LR, Ishikawa LL, Garces HG, Bagagli E, Differential behaviour of Non-albicansCandida species in the central nervous system of immunocompetent and immunosuppressed miceFront Microbiol 2018 9:296810.3389/fmicb.2018.0296830671026 [Google Scholar] [CrossRef] [PubMed]
[12]. Maqsood S, Badar F, Hameed A, Characteristics and outcomes of patients with haematological malignancies admitted for intensive care- A single centre experienceAsian Pac J Cancer Prev 2017 18(7):1833-37. [Google Scholar]