An Acute Pharyngeal-Cervical-Brachial Variant of Guillain-Barre Syndrome Manifesting as Isolated Bulbar Palsy
Ayan Husain1, Apoorva Nirmal2, Parag Aradhey3, Sourya Acharya4
1 Resident Doctor, Department of Medicine, Jawaharlal Nehru Medical College, Wardha, Maharashtra, India.
2 Resident Doctor, Department of Medicine, Jawaharlal Nehru Medical College, Wardha, Maharashtra, India.
3 Neurologist, Department of Medicine, Jawaharlal Nehru Medical College, Wardha, Maharashtra, India.
4 Professor, Department of Medicine, Jawaharlal Nehru Medical College, Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ayan Husain, Flat No. A-3, 2nd Floor, Vishwakarma Apts, Paloti Road, Sawangi-442001, Wardha, Maharashtra, India.
E-mail:ayanhusain123@gmail.com
Guillain Barre Syndrome (GBS) is characterised by acute onset ascending quadriparesis with areflexia. One of its rare variants is the Pharyngeal-Cervical-Brachial (PCB) variant which is characterised by acute weakness of the oropharyngeal, neck, and shoulder muscles with swallowing dysfunction. Here, the authors report a case of 57-year-old female, who presented with sudden onset dysphagia and nasal voice without having any limb weakness. Examination revealed reduced reflexes and weak palatal and pharyngeal muscles. Cerebrospinal Fluid (CSF) examination showed an albumino-cytological dissociation and nerve conduction studies revealed demyelinating motor polyneuropathy. A test for anti-GT1a antibody was positive. These findings were consistent with GBS. However, the patient presented only with bulbar involvement which is likely to be the milder form of the rare PCB variant. The patient recovered with plasmapheresis and was discharged in around three weeks. We conclude that GBS should be considered as differential diagnosis in a patient with isolated bulbar palsy.
Albumino-cytological dissociation, Anti-GT1a antibody, Dysphagia
Case Report
A 57-year-old female presented with a two day history of tingling sensation and numbness in both hands. She also noticed a nasal twang to her voice and difficulty in swallowing mainly to liquids and diplopia since one day. There was no limb weakness, unsteadiness, facial asymmetry, breathlessness or bowel/bladder involvement. She gave history of an upper respiratory tract infection four days prior to the development of these symptoms. She was a known case of type 2 diabetes mellitus on medications since 15 years. Her family history and personal history were insignificant and there was no history of injury/trauma.
On admission, she was afebrile and her blood pressure was 130/80 mmHg, and pulse rate 68/minute (regular). She was not in respiratory distress and had a Single Breath Count (SBC) of 26. A neurological examination showed marked bulbar palsy involving the pharyngeal and palatal muscles along with bilateral gaze paresis. There was no papilloedema. There was no weakness in facial muscles and neck flexors. In the upper limb muscles, power was grade 4+/5, the deep tendon reflexes were reduced, and plantar responses were flexor bilaterally. There was no sensory deficit. Neurological examination of the lower limbs was normal. Clinical examination of the lungs, abdomen and heart was normal.
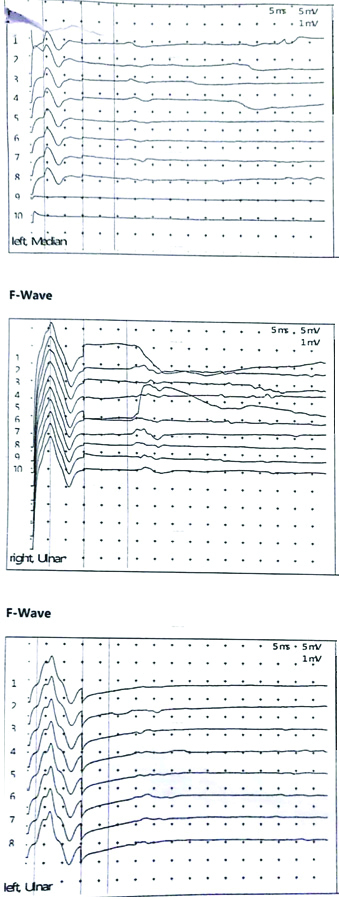
Her haemoglobin was 12.3 g%, white cell count 9700/cu.mm and platelet count 2.74 lac/cu.mm. The serum urea was 24 mg/dL, creatinine 0.5 mg/dL, sodium 139 mEq/L, potassium was 4.3 mEq/L, random blood glucose was 132 mmol/L and the liver function test was within normal limits. The chest radiograph was also normal. CSF examination showed albuminocytological dissociation with high protein level of 227 mg/L and only 2-3 cells/cumm. MRI brain and cervical spine was done to rule out structural pathology and it was normal. A repetitive nerve stimulation test was done which did not show decremental response, and anti-acetylcholine receptor (anti-AChR) antibody test was also normal. The nerve conduction studies revealed conduction block in left median, left ulnar and right ulnar nerves [Table/Fig-1,2a,b and c] with absent/dispersed F-wave latencies [Table/Fig-3]. The Sensory Nerve Action Potential (SNAP) and sensory latencies were normal. This was suggestive of demyelinating motor polyneuropathy consistent with GBS. Ganglioside antibody profile was done in which anti-GT1a antibody was positive and anti-GM1 antibody was negative.
Nerve conduction studies showing conduction block in left median and ulnar, and right ulnar nerves.

Motor conduction velocities in left median nerve showing conduction block.
| N | Stimulation site | Distance, mm | Latency, ms | Amplitude, mV | Duration, ms |
|---|
| 1 | Wrist | 80 | 3.5 | 5.7 | 3.5 |
| 4 | Axilla | 100 | | 0 | |
Motor conduction velocities in right ulnar nerve showing conduction block.
| N | Stimulation site | Distance, mm | Latency, ms | Amplitude, mV | Duration, ms | Velocity, m/s |
|---|
| 1 | Wrist | 80 | 2.6 | 13.2 | 7.1 | |
| 2 | Elbow | 260 | 7.8 | 9.8 | 7.2 | 50.1 |
Motor conduction velocities in left ulnar nerve showing conduction block.
| N | Stimulation site | Distance, mm | Latency, ms | Amplitude, mV | Duration, ms | Velocity, m/s |
|---|
| 1 | Wrist | 80 | 2.6 | 13.3 | 7.5 | |
| 2 | Elbow | 260 | 6.4 | 4.9 | 7.6 | 65.6 |
Nerve conduction studies showing prolonged F-wave latencies in left median and ulnar, and right ulnar nerves.
The clinical, CSF and electrophysiological findings lead to the diagnosis of GBS. A nasogastric tube was inserted and she was intubated on the 3rd day to prevent aspiration and put on T-piece. She was given five cycles of plasmapheresis. She was extubated by the end of the second week and the patient started to show marked improvement in terms of diplopia, swallowing difficulty and nasal voice, and the nasogastric tube was removed. By day 15, she could swallow solid and liquid food, reflexes had returned to normal by 3rd week and she was discharged on day 20.
Discussion
The PCB variant of GBS is characterised by acute weakness of the oropharyngeal, neck, and shoulder muscles with swallowing dysfunction [1]. The most common form of GBS is the Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP) type which is manifested by acute onset ascending quadriparesis with absence of deep tendon reflexes [2]. Other major forms are Acute Motor Axonal Neuropathy (AMAN) and Acute Sensori-Motor Axonal Neuropathy (ASMAN) [3,4]. The rarer phenotypic variants that have been recently described are Miller-Fisher Syndrome (MFS), the pure sensory variant, restricted autonomic manifestations and the PCB pattern [5,6].
In 1986, the rare PCB variant of GBS was first reported by Roper AH [6]. This variant differs from the AIDP type, that the leg strength and leg reflexes are usually preserved. The condition involved the bulbar muscles along with upper limbs and spared the lower limbs, so he called it PCB variant of GBS. Its incidence is approximately 0.07-0.25/100,000 [7]. The manifestations are severe bulbar palsy, slowly progressing to facial palsy, weakness of neck flexors and proximal muscles of upper limbs [8]. Acute GBS and its PCB variant share other diagnostic features- including areflexia, raised CSF protein, nerve studies revealing demylenating disease. PCB may also be seen after dengue fever and leptospirosis [1,9].
The index patient had no complaints of motor weakness in the upper limbs but clinical examination suggested a mild subclinical motor weakness in upper limbs. Neurophysiological study also gave evidence of conduction block and demyelination in median and ulnar nerves suggesting of demyelinating polyneuropathy. MRI brain was advised as lesions affecting the brainstem may present with isolated bulbar palsy but it showed no abnormality. A normal repetitive nerve stimulation test and anti-AChR antibody levels ruled out bulbar-onset myasthenia gravis which is a close differential diagnosis. Thus, there was a predominant bulbar muscle involvement which improved dramatically within two weeks. This variant is associated with the anti-GTla IgG antibody [10]. Thus, it might be a milder manifestation of the same immune-pathogenetic process as in the PCB variant.
Serum anti-GTla IgG antibody has been detected in about 50% of the PCB variant cases of GBS [11]. So far, there has been only two case reports showing isolated acute bulbar palsy due to the PCB variant of GBS [10,12]. The other cases did not have even the slightest of upper limb weakness on clinical examination but demyelinating polyneuropathy on nerve conduction studies was seen [12]. Both the cases had positive anti-GT1a antibody.
The index patient improved markedly after five sessions of plasmapheresis. Furthermore, there was no limb weakness and the respiratory muscles were not compromised. Nasogastric tube was inserted as she had bulbar muscle weakness. It is crucial during this period not to give oral feeding as this may cause aspiration pneumonia. The patient recovered soon and did not require intubation; while Percutaneous Endoscopic Gastrotomy (PEG) tube is required in patients with severe PCB variant of GBS.
Conclusion(s)
It can be concluded that GBS can rarely present as an isolated bulbar palsy. Hence, it should be ruled out in such a patient as it can rapidly improve with appropriate treatment.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 04, 2020
Manual Googling: Apr 15, 2020
iThenticate Software: Apr 24, 2020 (16%)
[1]. Pandey RK, Jain RK, Hussain SZ, Pharyngeal-cervical-brachial variant of Guillain-Barré syndrome following dengue infection: A rare syndrome with rare associationAnn Indian Acad Neurol 2019 22(2):240-41. [Google Scholar]
[2]. Dimachkie MM, Barohn RJ, Guillain-Barré syndrome and variantsNeurol Clinics 2013 31(2):491-510.10.1016/j.ncl.2013.01.00523642721 [Google Scholar] [CrossRef] [PubMed]
[3]. McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, Acute motor axonal neuropathy: A frequent cause of acute flaccid paralysis in ChinaAnn Neurol 1993 33(4):333-42.10.1002/ana.4103304028489203 [Google Scholar] [CrossRef] [PubMed]
[4]. Griffin JW, Li CY, Ho TW, Tian M, Gao CY, Xue P, Pathology of the motor-sensory axonal Guillain-Barré syndromeAnn Neurol 1996 39(1):17-28.10.1002/ana.4103901058572662 [Google Scholar] [CrossRef] [PubMed]
[5]. Fisher CM, An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia)N Engl J Med 1956 255(2):57-65.10.1056/NEJM19560712255020113334797 [Google Scholar] [CrossRef] [PubMed]
[6]. Ropper AH, Unusual clinical variants and signs in Guillain-Barre syndromeArch Neurol 1986 43:1150-52.10.1001/archneur.1986.005201100440122946281 [Google Scholar] [CrossRef] [PubMed]
[7]. E. Clinical, E. P. NeurologyGuillain-Barre syndrome variants in Emilia-Romagna, Italy, 1992-3: Incidence, clinical features, and prognosis. Emilia-Romagna Study Group on Clinical and Epidemiological Problems in NeurologyJ Neurol Neurosurg Psychiatry 1998 65(2):218-24.10.1136/jnnp.65.2.2189703176 [Google Scholar] [CrossRef] [PubMed]
[8]. Miura Y, Susuki K, Yuki N, Ayabe M, Shoji H, Guillain-Barre syndrome presenting as pharyngeal-cervical-brachia1 weakness in the recovery phaseEur Neurol 2002 48:53-54.10.1159/00006496312138316 [Google Scholar] [CrossRef] [PubMed]
[9]. Sherdiwala F, Gavhane J, Nathwani I, Patel A, Rewathi N, Patil S, Cervical-pharyngeal-brachial variant of Guillain-Barre Syndrome: A sequal of LeptospirosisMGM J Med Sci 2017 4(2):105-06.10.5005/jp-journals-10036-1150 [Google Scholar] [CrossRef]
[10]. Onodera M, Mori M, Koga M, Kamitsukasa I, Fukutake T, Hattori T, Acute isolated bulbar palsy with anti GTla IgG antibody subsequent to Campylobacter jejuni enteritisJ Neurol Sc 2002 205(1):83-84.10.1016/S0022-510X(02)00241-1 [Google Scholar] [CrossRef]
[11]. Wakerley BR, Yuki NJ, Pharyngeal-cervical-brachial variant of Guillain-Barre syndromeJ Neurol Neurosurg Psychiatry 2014 85(3):339-44.10.1136/jnnp-2013-30539723804237 [Google Scholar] [CrossRef] [PubMed]
[12]. Hamidon BB, An Acute Pharyngeal-Cervical-Brachial (PCB) variant of Guillain-Barre Syndrome presenting with isolated bulbar palsyMed J Malaysia 2006 61(2):245-47. [Google Scholar]