Endovascular Management in Post Liver Transplant Recipients with Venous Anastomotic Site Stenosis and an Associated Iatrogenic Arterio-portal Fistula: Case Series and Review of Literature
Raghunandan Prasad1, Rajanikant R Yadav2, Amrin Israrahmed3, Somit R Mittal4
1 Associate Professor, Department of Radiodiagnosis, SGPGIMS, Lucknow, Uttar Pradesh, India.
2 Associate Professor, Department of Radiodiagnosis, SGPGIMS, Lucknow, Uttar Pradesh, India.
3 Senior Resident, Department of Radiodiagnosis, SGPGIMS, Lucknow, Uttar Pradesh, India.
4 Senior Resident, Department of Radiodiagnosis, SGPGIMS, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Raghunandan Prasad, Type 4/74, SGPGIMS Campus, Raibareli Road, Lucknow, Uttar Pradesh, India.
E-mail: coolraghu2001@gmail.com
Venous anastomotic site stenosis is a relatively uncommon but an important complication seen in patients post-liver transplant surgery. These stenosis can occur at hepatic vein, portal vein or Inferior Vena Cava (IVC) anastomotic site and may result in hepatic congestion leading to hepatic dysfunction and massive ascites. Routine Doppler ultrasonography is critical for early detection of these complications and in guiding further management. With the advancement in interventional radiology, most of these complications can now be managed by minimally invasive endovascular methods with successful long term outcome. This case series of three patients (one cadaveric and two live related donor recipient) portrays the role of doppler ultrasonography in post-liver transplant graft surveillance and concludes that ultrasound plays a critical role in early diagnosis of venous anastomotic stenosis. The series also highlights the key role of interventional radiology in treating the stenosis safely and successfully by balloon angioplasty with or without stenting.
Balloon angioplasty, Doppler ultrasonography, Embolisation, Portal vein angioplasty
Case Series
Case 1
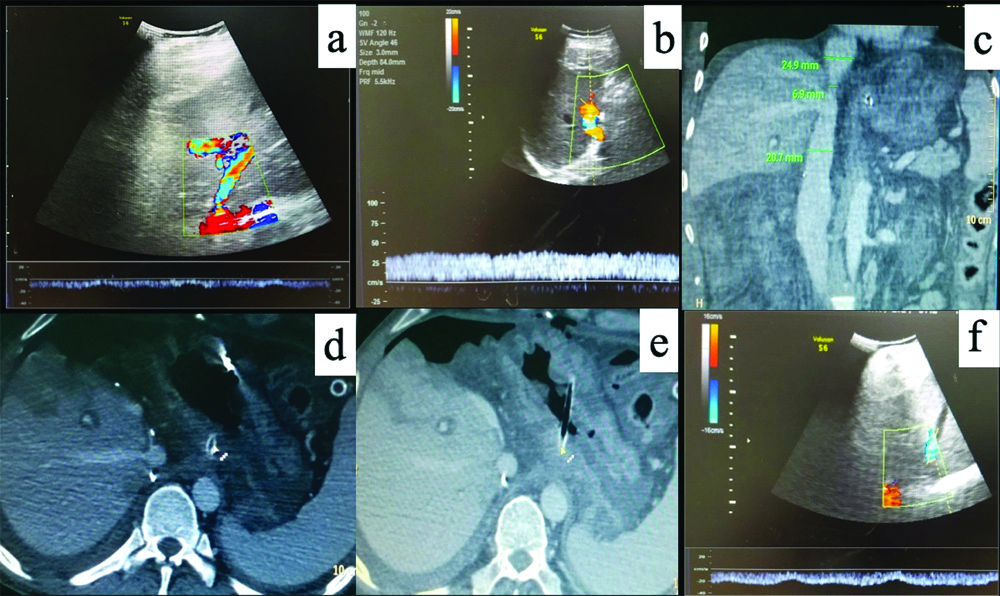
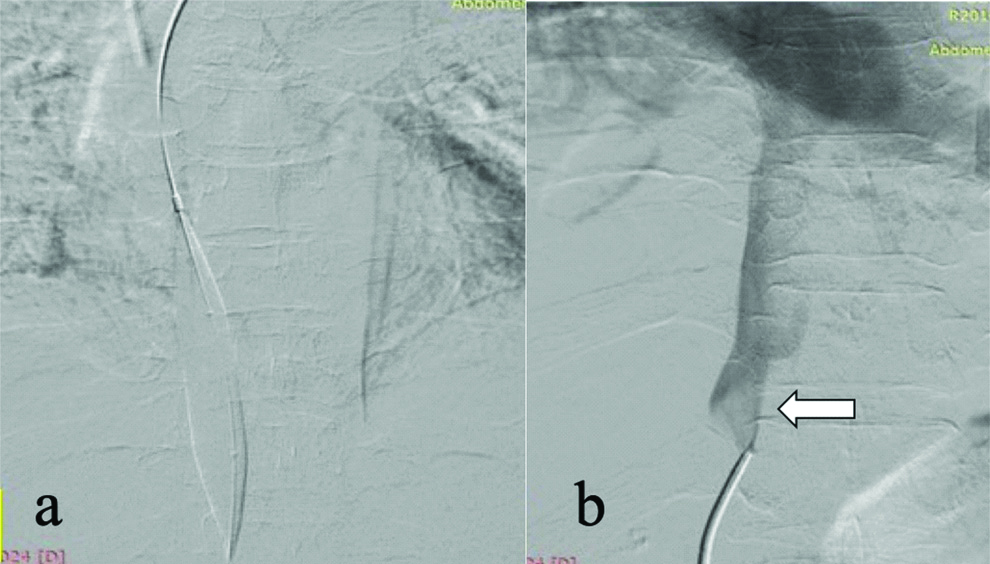
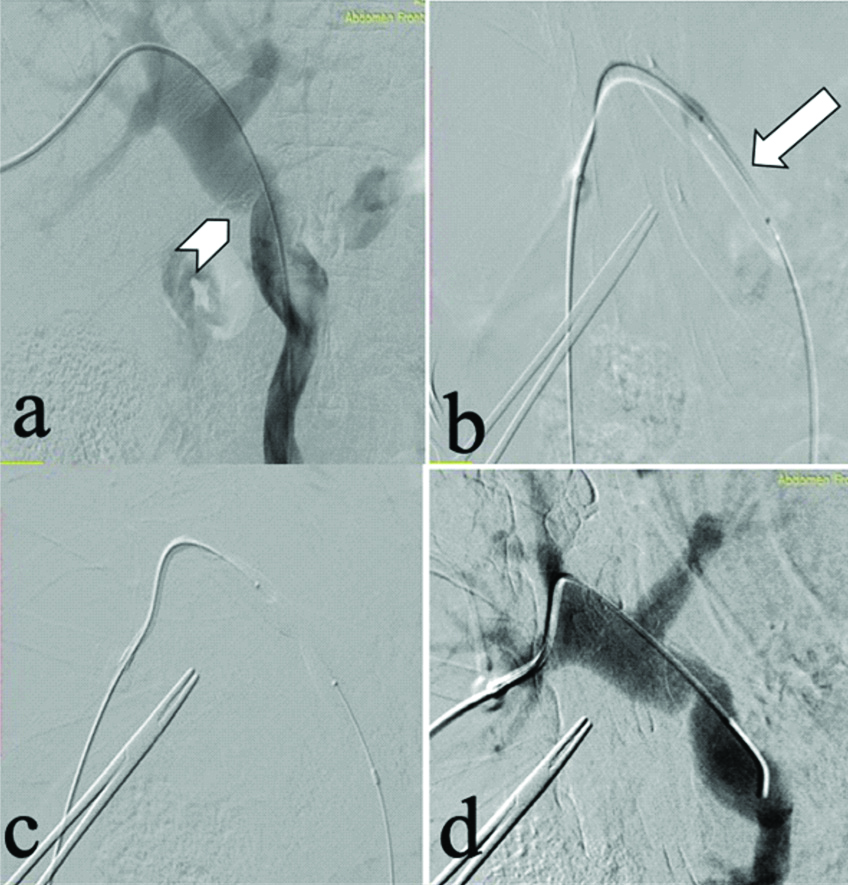
A 49-year-old male, who underwent orthotropic Liver Transplant (LT) for Non-Alcoholic Steatohepatitis (NASH) related decompensated Chronic Liver Disease (CLD), presented with ascites not responding to standard medications four months after LT. Doppler Ultrasound (D-USG) revealed mild narrowing of IVC in the anastomotic region (~10 mm) with dampened wave forms and loss of triphasic flow in the hepatic veins [Table/Fig-1]. Portal vein showed normal antegrade flow. The findings were further confirmed on Contrast Enhanced Computed Tomography (CECT) abdomen, which showed significant narrowing of the IVC at the anastomotic site with congestive changes in liver [Table/Fig-1c-e]. In view of refractory ascites, patient underwent Digital Subtraction Angiography (DSA) for a check venography with pressure gradient measurement across the stenosis. With help of a 10F (French) vascular access sheath in right femoral vein, IVC gram was taken followed by pressure gradient measurements in the supra-hepatic IVC and hepatic IVC across the anastomosis with the help of 5F Multipurpose Angled (MPA) catheter (Cook Medical, Bloomington, Indiana, USA), which was 12 mmHg. The stenosis was serially dilated with 12, 14 and 16 mm balloon dilatation catheter (Conquest or Atlas UHP balloon, Bard Peripheral Vascular, Inc, Tempe, Ariz) [Table/Fig-2]. Post-angioplasty, the pressure gradient reduced to normal limit (3 mmHg), hence stenting was not performed. D-USG showed restoration of biphasic flow in middle hepatic vein. At 6 months follow-up, he presented with recurrent ascites and D-USG showed partial stenosis at portal vein anastomotic site. At first the pressure across the IVC anastomosis was re-checked in DSA and confirmed to be normal. A percutaneous portal vein angioplasty with pressure gradient measurement was planned. A 5F vascular access was secured via transhepatic percutaneous approach in the segment 5 portal branch. Pre-dilatation pressure gradient in Main Portal Vein (MPV) was ~8 mmHg, which reduced to 2 mmHg after serial dilatation with 10 mm and 12 mm balloons (10% oversizing, 9 mm diameter of MPV) [Table/Fig-3] and percutaneous puncture tract was coil embolised.
(a) Pre-procedure colour doppler wave forms in middle hepatic vein show dampened monophasic pattern with aliasing suggestive of stenosis; (b) Normal anterograde flow in portal vein; (c) Computed Tomography (CT) angiogram venous phase coronal image show narrowing of the anastomotic segment of IVC. CT axial image in porto venous phase; (d) shows a hypo-enhancing area of liver parenchyma in segment VIII with delayed enhancement in late venous phase; (e) suggestive of liver congestion; (f) Post IVC stenting doppler shows restoration of biphasic wave forms in middle hepatic vein.
(a) IVC balloon angioplasty across anastomotic IVC stenosis; (b) IVC venogram showing normal forward flow (WhiteArrow) in IVC without stenosis.
(a) Percutaneous transhepatic cannulation angiogram shows main portal vein stricture (arrow head). Serial balloon dilatation; (b,c) (white arrow) was done with (10 mm, 12 mm); (d) Check angiogram showed minimal residual stenosis, however pressure gradient was reduced.
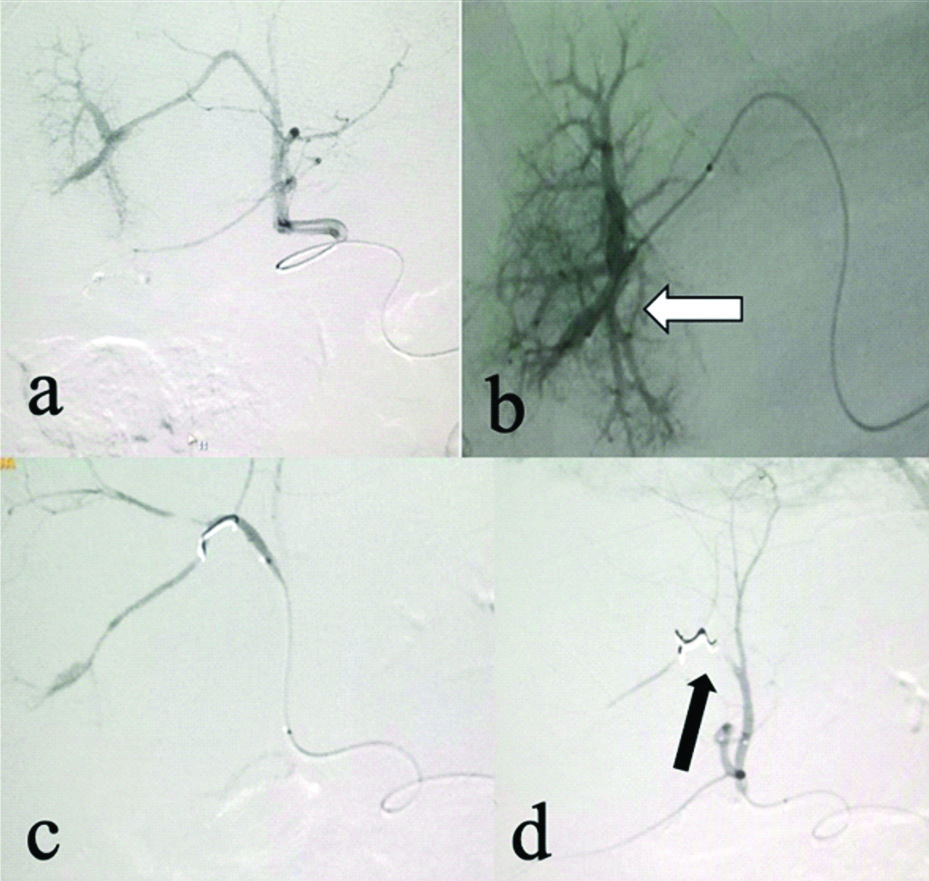
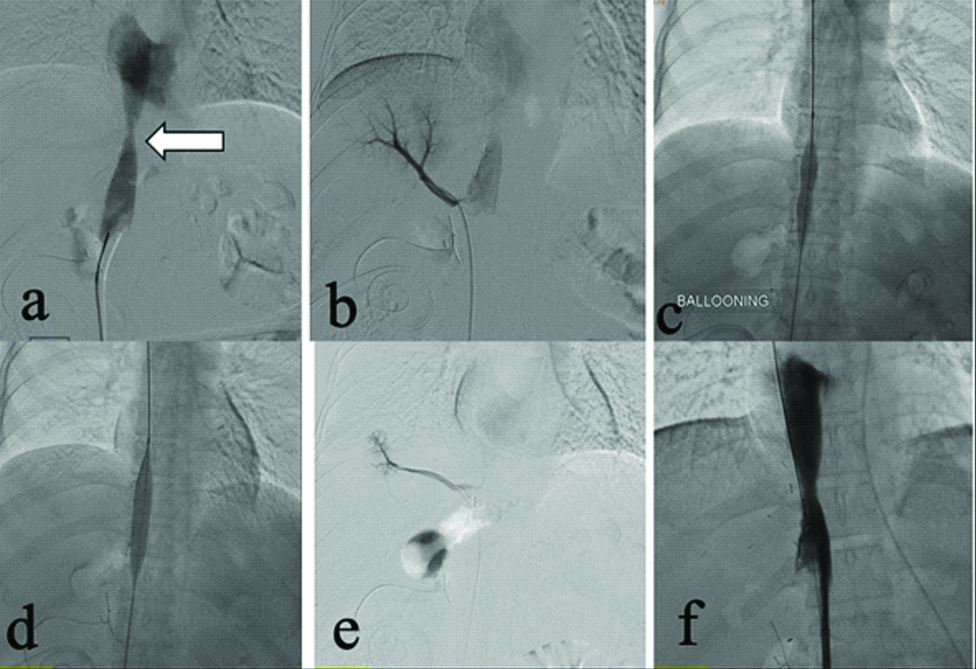
At 7-month post-orthotopic LT, on routine D-USG an iatrogenic Arterio-Portal Fistula (APF) was detected between segment 5 artery and corresponding portal vein. Endovascular coil embolisation of APF was done [Table/Fig-4-a,b]. Post-procedure angiogram [Table/Fig-4c,d] showed mild residual filling of portal vein from very fine feeders, however, these were not embolised to minimise graft ischaemia. Patient has completed five years of follow-up and is doing fine.
Selective celiac: (a) and super selective right hepatic artery angiogram; (b) reveals haemodynamically significant arterioportal shunting between right hepatic artery and anterior branch of right portal vein (white arrows); (c,d) PostCoil embolisation of culprit vessel (black arrows) check angiogram reveals partial residual APF getting supply from multiple non-defined feeders.
Case 2
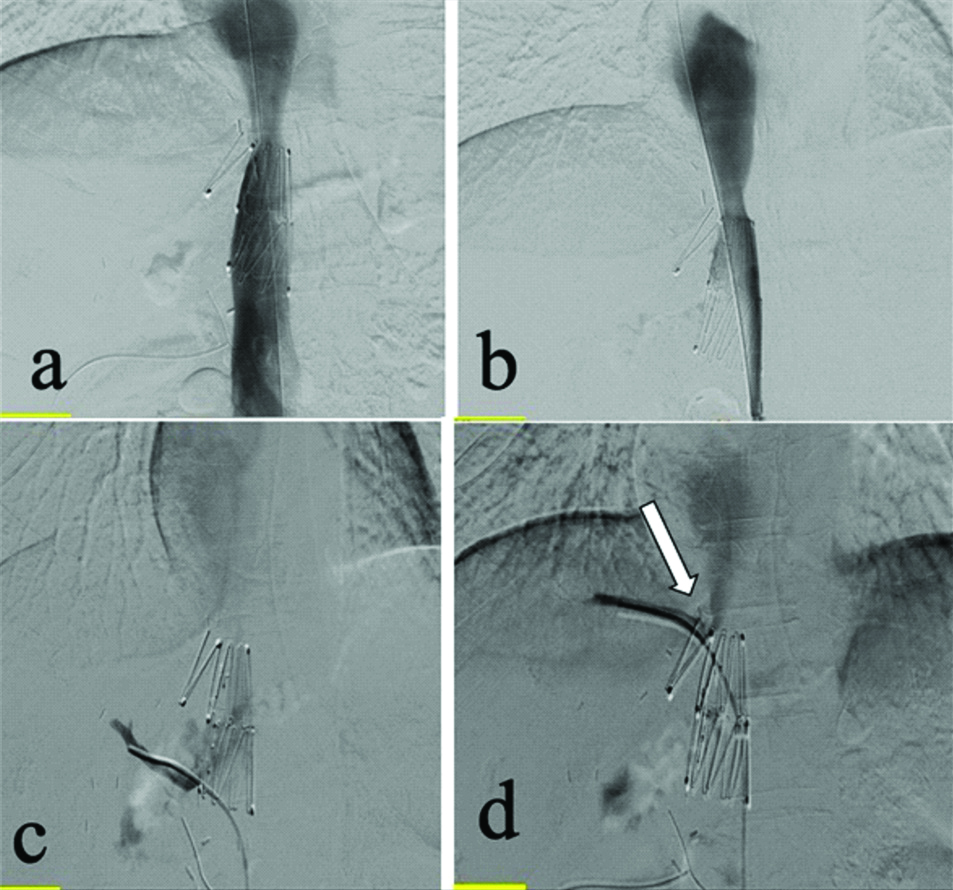
A 38 year old chronic alcoholic with CLD underwent Living Donor Liver Transplantation (LDLT). He had persistent ascites in the sixth month of follow-up resistant to medical treatment. D-USG abdomen revealed IVC stenosis at the anastomotic site, hence CECT abdomen was performed, which revealed mild IVC narrowing (~7.5 mm). IVC angioplasty was planned. A pressure gradient of 9 mmHg was recorded across the anastomotic site, and IVC angioplasty was done with serial dilatation of 10 and 12 mm diameter balloons. The pressure barely reduced to 7 mmHg, hence IVC stenting was decided [Table/Fig-5]. A self-expandable metallic stent of 24 mm diameter (Cook Z stent- Giantarco Rosch) was placed in the IVC avoiding the right hepatic vein ostium, following which the pressure gradient decreased to 2 mmHg. He has completed 4.5 years follow-up and is doing well clinically.
IVC gram with stent across the site of stenosis (a and b). Selective catheterization of right hepatic vein (white arrow) showed good forward flow with no evidence of stenosis across the IVC (c and d).
Case 3
A 47-year-old male, known case of alcohol related CLD, underwent LDLT. After three months, he developed refractory ascites. D-USG revealed stenosis at the IVC anastomotic site. IVC angioplasty with documentation of pressure gradient was planned in DSA. A pressure gradient of 7 mmHg was recorded across the anastomotic site; hence IVC angioplasty was done serially with (10 and 12 mm diameter) balloons [Table/Fig-6]. Post plain balloon IVC angioplasty, the pressure gradient reduced to 2 mmHg. Patient was discharged after four days with no complaints and has completed 4 years of follow-up.
IVC gram (a) and Right hepatic vein venogram (b) showed evidence of IVC narrowing (white arrow) which was dilated with balloon (12 mm diameter) (c and d). Check venogram (e and f) shows no residual stenosis with good forward flow.
Discussion
Hepatic Vein and IVC Stenosis
Venous anastomotic site stenosis is an uncommon complication after the liver transplant surgery but requires prompt diagnosis and immediate intervention. With evolving times, endovascular interventions have played a significant role in reducing the morbidity and mortality of these post-LT patients [1]. These stenosis may occur at hepatic vein, portal vein or IVC anastomotic site and result in hepatic congestion leading to hepatic dysfunction and ascites [2]. In this case series too the first patient had a similar clinical and CT picture with areas of hepatic congestion. The incidence of hepatic vein stenosis varies from 5-13% with one series of 152 LDLT patients, reporting it to be 5.3% and another series mentioning it to be upto 12.9% in reduced sized livers [3,4]. It’s common occurrence in paediatric liver transplants can be explained by the twisting of small graft around the HV anastomosis [4]. Orons PD et al., in their study have reported Hepatic arterial thrombosis and stenosis as the most common vascular complications (~4-25%) followed by portal venous, hepatic venous and IVC stenosis (~3%) in patients receiving LT [5].
Patients with Hepatic vein or IVC stenosis commonly present with hepatic dysfunction, ascites, lower extremity oedema or they be asymptomatic [6]. However, early identification and management of these vascular complications are very important to salvage the graft and to avoid severe complications like portal hypertension, hepatic necrosis, haemorrhage, liver failure and even death [7-12]. Routine screening with doppler is critical and in many cases sufficient for early detection of these complications [13]. There are many techniques which are used for hepatic vein and IVC reconstruction during LT and in-depth knowledge of these techniques is very important while doing doppler ultrasound of these liver transplant patients or planning the route of endovascular treatment. Two commonly used techniques of surgery are conventional and piggy back techniques. In conventional technique-donor’s hepatic IVC is harvested along with the graft liver and is interposed between the recipient supra-hepatic and hepatic/infra-hepatic IVC with end-to-end anastomosis, hence risk of stenosis is usually at either ends of caval anastomosis. In piggyback technique, the supra-hepatic donor IVC is anastomosed to the recipient hepatic vein in end to end manner with total ligation of infra-hepatic end of donor IVC, hence here the stenosis of the hepatic vein origin is common. In LDLT patients, hepatic vein origins are dissected free and graft hepatic veins are anastomosed with cuff of recipient’s hepatic veins in end to end manner [14].
Most of the HV occlusions can be identified on routine D-USG. The normal hepatic venous flow is triphasic due to transmission of cardiac pulsations from the heart, and in cases of HV stenosis, this tri-phasic flow pattern is lost. Although the presence of triphasic flow has good sensitivity for excluding hepatic vein stenosis, however, absence of triphasic waveform does not mean the presence of HV stenosis, as this finding is commonly seen in post-operative LT patients [13].
In scenarios where the D-USG cannot completely exclude HV stenosis, IVC venography and pressure measurements across the anastomosis are of critical importance and are considered gold standard for diagnosis [15]. A pressure gradient of >10 mmHg across stenosis makes it haemodynamically significant, although few studies have shown satisfactory results with treating patients having a gradient of 3 mmHg [1]. The fibrotic IVC lesions are easily treated with balloon angioplasty; however for recurrent or compliant lesions stenting is the treatment of choice [16]. Occasionally, with tight venous anastomosis, percutaneous trans-hepatic approach can be considered. Parvinian A and Gaba RC, have described how a recalcitrant stenosis in post-LT patient, was not responsive to single session of IVC balloonplasty. However, he showed dramatic improvement on repeat session of serial dilatation of balloon angioplasties thus, highlighting the role of programmed approach to balloon venoplasty with multiple sessions [17]. Similarly, in case 1 and case 3 serial balloon dilatations helped in reducing the pressure gradient and thereby in clinical improvement of the patients. A recent study mentioned the effectiveness of IVC stenting in post-LT patients showing 100% technical success, 85% clinical success and 96% of stents showed long term patency [18]. Lee JM et al., have discussed the long-term patency of IVC stenting in fourteen post-LT patients, wherein elastic restenosis of more than 50% or a pressure gradient of more than 5 mm Hg, or failure to show clinical improvement following balloon angioplasty was established as criteria for IVC stenting [19]. Weeks SM et al., described that initial balloon angioplasty failed in 13 of 16 patients who later underwent stent placement in their literature review of a total of forty-three patients who had IVC abnormalities post-LT [20]. Thus, we can compare these scenarios to case 2 which showed no improvement to serial balloon dilatations and hence IVC stenting had to be done which resulted in significant drop in pressure gradient and symptomatic improvement of the patient.
PV Stenosis
Values higher than 5 mm Hg are considered significant for portal vein stenosis [6]. Percutaneous Transluminal Angioplasty is (PTA) the treatment of choice with a success rate of ~70% and stenting is usually reserved for resistant or recurrent stenotic lesions [21,22].
Arterio-Portal Fistula
Iatrogenic APF commonly occurs due to liver biopsies [23,24]. APF show low resistance pattern in the supplying artery and arterial flow in the portal vein on D-USG. Surgical ligature is not a practical option as it involves extensive dissection and hence endovascular embolisation with micro coils, N-butyl cyanoacrylate and detachable balloons is the treatment of choice to prevent the risk of graft infarction [25,26].
Conclusion(s)
D-USG plays a critical role in surveillance of Liver transplant recipients by helping in early detection of vascular complications, most of which can be managed successfully and safely by Endovascular techniques. However, a good knowledge of the surgical technique utilised for transplantation is of utmost importance for doppler evaluation and planning the appropriate endovascular therapy.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 05, 2020
Manual Googling: Apr 14, 2020
iThenticate Software: Apr 22, 2020 (1%)
[1]. Thornburg B, Katariya N, Riaz A, Desai K, Hickey R, Lewandowski R, Interventional radiology in the management of the liver transplant patientLiver Transpl 2017 23(10):1328-41.10.1002/lt.2482828741309 [Google Scholar] [CrossRef] [PubMed]
[2]. Yang J, Xu MQ, Yan LN, Lu WS, Li X, Shi ZR, Management of venous stenosis in living donor liver transplant recipientsWorld J Gastroenterol 2009 15(39):4969-73.10.3748/wjg.15.496919842231 [Google Scholar] [CrossRef] [PubMed]
[3]. Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, Okajima H, Hepatic vein reconstruction in 152 living-related donor liver transplantation patientsSurgery 1997 121(3):250-57.10.1016/S0039-6060(97)90353-6 [Google Scholar] [CrossRef]
[4]. Emond JC, Heffron TG, Whitington PF, Broelsch CE, Reconstruction of the hepatic vein in reduced size hepatic transplantationSurg Gynecol Obstet 1993 176(1):11-17. [Google Scholar]
[5]. Orons PD, Zajko AB, Bron KM, Trecha GT, Selby RR, Fung JJ, Hepatic artery angioplasty after liver transplantation: Experience in 21 allograftsJ Vasc Interv Radiol 1995 6(4):523-29.10.1016/S1051-0443(95)71128-9 [Google Scholar] [CrossRef]
[6]. Glockner JF, Forauer AR, Vascular or ischemic complications after liver transplantationAm J Roentgenol 1999 173(4):1055-59.10.2214/ajr.173.4.1051117710511177 [Google Scholar] [CrossRef] [PubMed]
[7]. Harihara Y, Makuuchi M, Takayama T, Kawarasaki H, Kubota K, Matsuura A, Venoplasty of recipient hepatic veins in living-related liver transplantationTransplant Proc 1998 30(7):320510.1016/S0041-1345(98)00995-6 [Google Scholar] [CrossRef]
[8]. Ko GY, Sung KB, Yoon HK, Kim JH, Song HY, Seo TS, Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantationJ Vasc Interv Radiol 2002 13(6):591-99.10.1016/S1051-0443(07)61652-2 [Google Scholar] [CrossRef]
[9]. Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Long-term venous complications after full-size and segmental pediatric liver transplantationAnn Surg 2002 236(6):658-66.10.1097/00000658-200211000-0001712409673 [Google Scholar] [CrossRef] [PubMed]
[10]. Kubo T, Shibata T, Itoh K, Maetani Y, Isoda H, Hiraoka M, Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantationRadiology 2006 239(1):285-90.10.1148/radiol.239105038716567488 [Google Scholar] [CrossRef] [PubMed]
[11]. Settmacher U, Nussler NC, Glanemann M, Haase R, Heise M, Bechstein WO, Venous complications after orthotopic liver transplantationClin Transplant 2000 14(3):235-41.10.1034/j.1399-0012.2000.140309.x10831082 [Google Scholar] [CrossRef] [PubMed]
[12]. Egawa H, Tanaka K, Uemoto S, Someda H, Moriyasu F, Sano K, Relief of hepatic vein stenosis by balloon angioplasty after living-related donor liver transplantationClin Transplant 1993 7(4):306-11. [Google Scholar]
[13]. Sanyal R, Zarzour JG, Ganeshan DM, Bhargava P, Lall CG, Little MD, Postoperative doppler evaluation of liver transplantsIndian J Radiol Imaging 2014 24(4):360-66.10.4103/0971-3026.14389825489129 [Google Scholar] [CrossRef] [PubMed]
[14]. Neuhaus P, Platz KP, Liver transplantation: Newer surgical approachesBaillieres Clin Gastroenterol 1994 8(3):481-93.10.1016/0950-3528(94)90033-7 [Google Scholar] [CrossRef]
[15]. Ferro C, Andorno E, Guastavino A, Rossi UG, Seitun S, Bovio G, Endovascular treatment with primary stenting of inferior cava vein torsion following orthotopic liver transplantation with modified piggyback techniqueLa Radiologia Medica 2013 119(3):183-188.10.1007/s11547-013-0325-424356944 [Google Scholar] [CrossRef] [PubMed]
[16]. Averin K, Bucuvalas J, Alonso MH, Kohli R, Heubi JE, Johnson ND, Treatment of inferior vena cava obstruction following pediatric liver transplantation: Novel use of a customized endovascular stentJ Pediatr 2017 180:256-60.10.1016/j.jpeds.2016.09.05127793336 [Google Scholar] [CrossRef] [PubMed]
[17]. Parvinian A, Gaba RC, Sequential venoplasty for treatment of inferior vena cava stenosis following liver transplantJ Clin Imaging Sci 2014 4:5010.4103/2156-7514.14155725337436 [Google Scholar] [CrossRef] [PubMed]
[18]. Donaldson J, Obuchowski NA, Le RT, Lomaglio L, Unger RH, Bayona MDP, Stenting for inferior vena cava stenosis after liver transplantAm J Roentgenol 2019 213(6):1381-87.10.2214/AJR.18.2091531573847 [Google Scholar] [CrossRef] [PubMed]
[19]. Lee JM, Ko GY, Sung KB, Gwon DI, Yoon HK, Lee SG, Long term efficacy of stent placement for treating inferior vena cava stenosis following liver transplantationLiver Transpl 2010 16(4):513-19.10.1002/lt.2202120213830 [Google Scholar] [CrossRef] [PubMed]
[20]. Weeks SM, Gerber DA, Jaques PF, Sandhu J, Johnson MW, Fair JH, Primary Gianturco stent placement for inferior vena cava abnormalities following liver transplantationJ Vasc Interv Radiol 2000 11(2):177-87.10.1016/S1051-0443(07)61462-6 [Google Scholar] [CrossRef]
[21]. Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM, Angioplasty treatment of portal vein stenosis in children with segmental liver transplants: Mid-term resultsAm J Roentgenol 1997 169(2):551-54.10.2214/ajr.169.2.92427759242775 [Google Scholar] [CrossRef] [PubMed]
[22]. Olcott EW, Ring EJ, Roberts JP, Ascher NL, Lake JR, Gordon RL, Percutaneous transhepatic portal vein angioplasty and stent placement after liver transplantation: Early experienceJ Vasc Interv Radiol 1990 1(1):17-22.10.1016/S1051-0443(90)72496-7 [Google Scholar] [CrossRef]
[23]. Chavan A, Harms J, Pichlmayr R, Galanski M, Transcatheter coil occlusion of an intrahepatic arterioportal fistula in a transplanted liverBildgebung 1993 60(4):215-18. [Google Scholar]
[24]. Lee SJ, Lim JH, Lee WJ, Lim HK, Choo SW, Choo IW, Transient subsegmental hepatic parenchymal enhancement on dynamic CT: A sign of postbiopsy arterioportal shuntJ Comput Assist Tomogr 1997 21(3):355-60.10.1097/00004728-199705000-000049135640 [Google Scholar] [CrossRef] [PubMed]
[25]. Redmond PL, Kumpe DA, Embolisation of intrahepatic arterioportal fistula: Case Report and review of the literatureCardiovasc Intervent Radiol 1998 11(5):274-77.10.1007/BF025770343145140 [Google Scholar] [CrossRef] [PubMed]
[26]. Saad WE, Arterioportal fistulas in liver transplant recipientsSemin Intervent Radiol 2012 29(2):105-10.10.1055/s-0032-131257123729980 [Google Scholar] [CrossRef] [PubMed]