Materials and Methods
This hospital based prospective study was carried out in the Department of Microbiology, IMS & SUM Hospital, Bhubaneswar, Odisha, India over a period of one and half year (January 2018-June 2019) after obtaining ethical clearance from institutional ethical committee (Ref No/DMR/IMS-SH/SOA/16075). After taking informed consent, the blood samples from 8156 ICU patients, clinically suspected to have septicaemia, were collected following all the aseptic precautions and before administration of antibiotics or antifungals and were screened for candidemia. All the Candida isolates obtained as a single pathogen from blood cultures were included in the study. Patients other than those of ICU admitted and also those who were under antibiotics or antifungals even from ICU admission status were excluded from this study.
A total of 8156 specimens were inoculated into the BacT/ALERT (BioMerieux) blood culture bottles and incubated at 37°C for a maximum period of seven days in BacT/ALERT 3D system, a fully automated blood culture system for detection of aerobic growth in blood samples and if there was no growth, the result was read as negative. From culture bottles which were flagged positive in the system, Gram stain was performed. If yeast cells were observed on staining, blood was subcultured on two Sabouraud’s Dextrose Agar (SDA) and blood agar plates and incubated at 37°C. SDA and blood agar plates were examined for white to cream coloured, smooth and pasty colonies after 24-48 hours of incubation [14].
Gram staining was performed from the colony and the morphology of yeast cells was noted. All the isolates were processed by both conventional and automated methods in parallel.
Colonies from the SDA were then plated onto cornmeal agar with Tween 80 (Dalmau plate culture) for chlamydospores production [15]. Germ tube test was performed from colonies for presumptive identification of C. albicans [16].
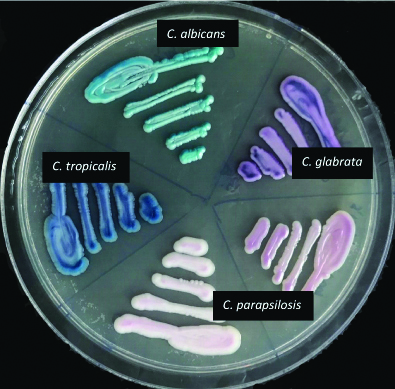
Colonies from SDA were plated onto CHROM agar (HiCrome Candida differential agar- Himedia), a differential culture medium that is claimed to facilitate the isolation by colorimetric presumptive identification [17] and were incubated at 37°C for 48 hours. The results were interpreted according to the colour of the colony formed and as per manufacturer’s guidelines [Table/Fig-1,2]. All the isolates that gave doubtful morphology or not identified by conventional methods were taken as “Candida spp.”
Appearance of various Candida species on CHROMagar.
| Candida species | Morphology on CHROM agar |
|---|
| C. albicans | Apple green |
| C. dubliniensis | Dark green |
| C. tropicalis | Metallic blue with a pink halo |
| C. parapsilosis | White to pale pink |
| C. glabrata | Pale pink to violet |
| C. krusei | Fuzzy pink coloured colonies with matt surface and white edges |
Colour production of various Candida species on CHROMagar.
In parallel to conventional methods listed above, for all the isolates identification and antifungal susceptibility testing were also done by Vitek-2 (BioMerieux) with 0.5% McFarland suspension from the colonies from SDA using the Identification Card-Yeast (ID-YST) and Antifungal Susceptibility Testing card for yeast (AST YS01) as per Clinical and Laboratory Standards Institute (CLSI) guidelines and manufacturer’s instructions [12]. Finally, the results of the two methods (conventional and Vitek-2) were compared with each other. Candida albicans ATCC 90028 strain was used as control for evaluation of various methods [18].
Statistical Analysis
It was done as per Chi-square test to compare the conventional and the automated method.
Results
A total of 100 non-duplicate isolates of Candida species were obtained from 2202 positive blood culture cases as per inclusion criteria during the study period.
Male patients (55/100, 55%) predominated the female patients (45/100, 45%) in terms of isolation frequency. Maximum number of candidemia patients were in the elderly age group (>60 years, mean age- 71), i.e., 43%, followed by 41-60 years (mean age- 51.3) (33%); 14% and 10% patients were seen in the age groups of 21-40 years (mean age- 32) and up to 20 years (mean age- 8.4), respectively.
Overall 60% of cases had associated co-morbid conditions like malignancy (29/100), kidney disease (40/100), indwelling vascular catheters (47/100), surgical interventions (26/100) and diabetes mellitus (52/100), etc.
Candida parapsilosis (26/100, 26%) was the predominant species causing candidemia followed by Candida tropicalis (23/100, 23%), Candida albicans (21/100, 21%), Candida auris (15/100, 15%), Candida glabrata (5/100, 5%) and others (10/100, 10%) [Table/Fig-3].
Comparison of Candida spp. identification by conventional and automated methods.
| Manual identification | Vitek 2 identification | |
|---|
| C. albicans | C. tropicalis | C. parapsilosis | C. glabrata | C. auris | C. guillermondi | C. utilis | C. famata | C. pelliculosa | C. haemulonii | C. rugosa | Total |
|---|
| C. albicans | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 |
| C. tropicalis | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| C. parapsilosis | 0 | 0 | 26 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 35 |
| C. glabrata | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Candida spp. | 0 | 0 | 0 | 0 | 7 | 2 | 3 | 1 | 1 | 2 | 1 | 17 |
| Total | 21 | 23 | 26 | 5 | 15 | 2 | 3 | 1 | 1 | 2 | 1 | 100 |
NB-concordance rate between two methods- 74/100
For speciation, of the total 100 Candida isolates concordance between conventional and Vitek-2 method was 74 (74%). Of the total C.parapsilosis identified by conventional method, 1 isolate as C. glabrata and 8 isolates as C. auris was established by Vitek-2 method. 17 isolates of Candida which could not be identified by conventional chromogenic method were interpreted as Candida spp. but final identification was considered as per Vitek-2 results. Vitek-2 is the better method (p<0.001) than the conventional method for Candida species identification.
Vitek-2 Compact system does not give Antifungal susceptibility test results for C.auris and C.famata, so Antifungal susceptibility rates were calculated for 84 isolates (n=84). Antifungal susceptibility rate for Amphotericin B, Caspofungin, Fluconazole, Flucytosine, Micafungin and Voriconazole was 94%, 90.5%, 73.8%, 96.4%, 98.8% and 95.2%, respectively [Table/Fig-4].
Antifungal susceptibility testing rate of various antifungals.
| Antifungals | Sensitive | Percentage |
|---|
| Amphotericin B | 79/84 | 94% |
| Caspofungin | 76/84 | 90.5% |
| Fluconazole | 62/84 | 73.8% |
| Flucytosine | 81/84 | 96.4% |
| Micafungin | 83/84 | 98.8% |
| Voriconazole | 80/84 | 95.2% |
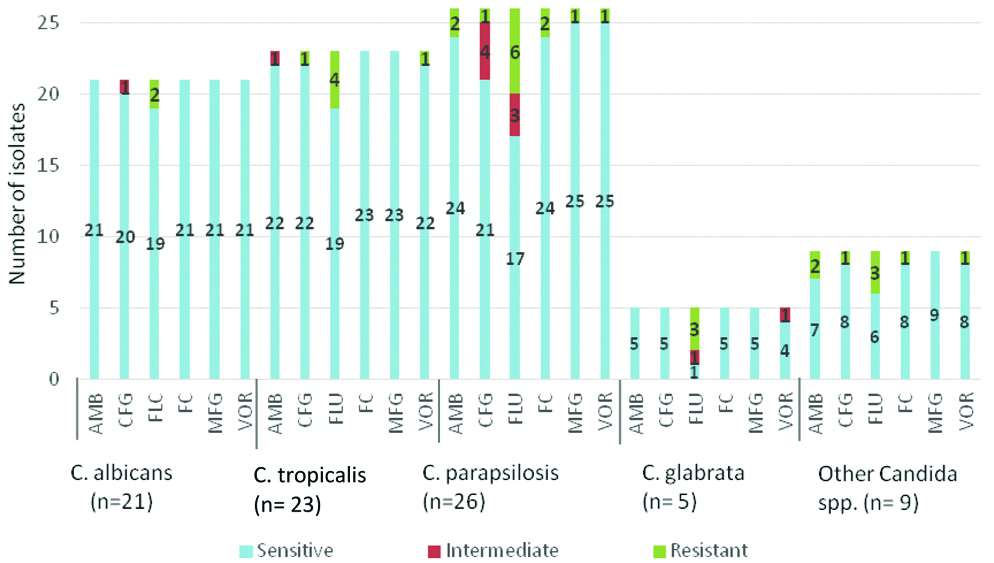
Different Candida species also showed variable sensitivity result to different antifungal drugs used [Table/Fig-5].
Antifungal susceptibility testing result for different Candida spp.
Mortality rate was found to be 45% due to sepsis and associated complications, 11% of patients were lost for follow-up and rest of the cases were treated successfully.
Discussion
Candidemia is an emerging problem in healthcare settings, particularly among those in ICU’s. Early isolation, speciation and antifungal susceptibility is essential for the choice of the best therapeutic approach for the patient, thereby, decreasing morbidity and mortality. Therefore, knowledge of epidemiology of candidemia can help to salvage patients. In this study, an overview of candidemia was obtained, including Candida species identification, antifungal susceptibility pattern, epidemiological characteristics and patient outcome.
In this study, candidemia was most commonly seen in patients >60 years of age (43%) which is in concordance with other studies [19,20]. On the contrary, a study by Thomas T and Dias M shows a higher preponderance in middle age groups (18-50 years) [9]. This was possibly because present study mainly focused on the critically ill patients in ICU’s. In this study, the common risk factors for candidemia were malignancy, diabetes mellitus and chronic kidney disease which is similar to a study by Thomas T and Dias M [9].
The isolation rate of candidemia in the present study was 4.54% which is in concordance with many other studies from India [Table/Fig-6] [5,14,18,21-23].
Studies showing incidence of candidemia from different parts of India [5,14,18,21-23].
| Author | Year | Area | Candidemia rate |
|---|
| Verma AK et al., [5] | 2003 | North India | 1.61% |
| Giri S et al., [18] | 2013 | South India | 5.76% |
| Chander J et al., [21] | 2013 | North India | 5.79% |
| Sudan SS et al., [22] | 2016 | J&K | 4.48 |
| Bhattacharjee P [23] | 2016 | Kolkata | 4.03% |
| Gandham NR et al., [14] | 2016 | Pune | 14.8% |
In the present global scenario, there is a changing trend of Candida species, with predominance of NAC spp. In the present study C. parapsilosis (26%) was isolated as the predominant isolate which is similar to a study by Van Schalkwyk E et al., [8], while some other studies had C. tropicalis [9,24] or C. albicans [2] as the predominant pathogen.
C. auris has surpassed the number of cases caused by C. albicans or NAC for the last 10 years and is a major cause of candidemia. In this study also, it was the fourth most common isolated species comprising 15% of candidemia cases. It was identified by Vtek-2 method though which has certain limitation in identification. C.auris identification is confirmed if the initial identification of C.auris is made and C.auris identification is possible if the initial identification of C.haemulonii, C.duobushaemulonii, C.famata or C.lusitaniae is made [25]. There have been reports of C. famata being misdiagnosed by Vitek-2 [26]. So, the ideal method of identification of such species is by Matrix Assisted Laser Desorption Ionisation-Time Of Flight Mass Spectrometry (MALDI-TOFMS). This multidrug resistant pathogen is known to cause protracted healthcare associated outbreaks. This possibly happens due to its ability to persist on surfaces, form biofilms and resist routinely used environmental cleansing agents. Similarly, in this study, C.auris was associated with micro-outbreaks. Presently, disinfectants having sporicidal activity or hydrogen peroxide- based products has been recommended to clean the surfaces in rooms of patients infected or colonised with C.auris because of its high efficiency in eliminating this species from inert surfaces [27,28].
Candida species differ in their susceptibility to antifungal agents. For instance, C.glabrata is not very sensitive to Fluconazole, C.krusei is intrinsically resistance to Fluconazole and C.lusitaniae is resistant to amphotericin B [29]. In this study, all antifungal agents except Fluconazole demonstrated excellent activity against all Candida species. Fluconazole being a cheaper drug has been used as main drug against Candida infections. So, the use of this drug should be as per susceptibility result. Similar findings were published in a study from Southern India [30].
Death rate by candidemia is quite high. According to some studies, invasive infection with Candida is associated with mortality rates of 35-80% in ICU settings [31-33]. The mortality rate of patients with candidemia in this study was 45% which is well within the range reported by other studies and even more concern is about auris which showed 100% mortality.
Candida speciation by conventional methods has long been used as benchmark identification procedures. However, these methods are time consuming, laborious and not reliable in identifying broad spectrum of species and require additional tests like assimilation and fermentation. Several commercial systems were developed which can produce rapid yeast identification and their antifungal susceptibility in at least 15 hour. Their use is limited because these systems are not reliable for identification of some species and some are also misidentified. So, now-a-days, molecular strategies, PCR or non-PCR based methods, are being used as complementary to conventional methods for providing more accurate results in less time.
Limitation(s)
Limitation of present study is that we have not done fermentation and assimilation tests which are the crucial means of species identification by conventional method, also considered as reference method. Although Vitek-2 is a reliable method for identification of common fungal species, for accurate speciation of C.auris, C.guilliermondii, C.haemulonii, C.famata, etc., molecular studies like nucleic acid sequencing may be required as researchers are now claiming Vitek-2 as the inaccurate method for identification of such species.
Conclusion(s)
Even though CHROMagar helps with identification at a lower cost as compared to Vitek-2, which is useful in countries having low resources, but it takes longer duration for complete identification, which is the main drawback of this method. Vitek-2 is considered as a reliable technique for antifungal susceptibility of yeast species, it also has the added advantage of being more rapid and easier than the alternative procedure developed by CLSI, broth microdilution method which is cumbersome and expensive. So, a fast and accurate technique for yeast identification is very important for microbiological laboratories. Accordingly, Vitek-2 can be applied for early identification and antifungal susceptibility testing. Local epidemiological data and antifungal susceptibility profile should be taken into consideration when establishing antifungal treatment strategies. Infection control measures like hand and personal hygiene by healthcare workers, proper catheter care, frequent clinical examination of patients who are weaned off invasive device to be practiced to reduce nosocomial transmission as candidemia represents 10% of nosocomial infections in hospitalised patients and also antibiotic stewardship must be emphasised.
NB-concordance rate between two methods- 74/100