Breast cancer is the most commonly diagnosed cancer in women (24.2%) worldwide. The worldwide incidence of breast malignancy is 11.6% and 5 year prevalence is 36%. It is the fifth common cause of death worldwide [1].
Breast cancer is a diverse disease in terms of presentation, morphology and molecular profile [2]. Genetic changes associated with transitions from normal epithelium to hyperplasia, and ultimately carcinoma, involve a series of events referred to as initiation, transformation, and progression [3]. Hence, the identification of involved premalignant lesions is very essential in preventing progression to development of breast cancer [4].
Breast tissue is heterogeneous and complex in composition. It is composed of epithelium, stroma, and adipose tissue. Each normal constituent of a breast is a source of both benign and malignant lesions [5]. There are important shifts which occur during critical developmental windows, like embryogenesis, pregnancy and postmenopausal involution [5,6]. With hormonal exposure epithelial proportions increase [7-9] and as malignant transformation evolves, composition of benign breast tissue may also change in parallel [10-13] which exerts selective forces, influencing the tumour biology and behaviour [14,15].
Benign Breast Diseases (BBDs) become more common during the second decade of life and peak during the fourth and fifth decades [16]. It had been observed that about 30% of all breast cancers develop in women with prior BBD [17]. Fibroadenoma and fibrocystic changes are the two most common BBDs [18-20]. Although non-proliferative disease does not appear to be associated with increased breast cancer risk [21], proliferative disease without atypia and that with atypia (ductal carcinoma in-situ, atypical hyperplasia, and sclerosing adenosis) have been associated with a 1.5- to 4-fold increased risk for breast cancer, respectively [22-24].
It is important for pathologists, radiologists, and oncologists to distinguish benign breast lesions from in-situ and invasive breast cancer and recognise them for assessing a patient’s risk of developing breast cancer. So, that the most appropriate treatment modality for each case can be established [22]. However, in many cases, the differentiation between benign and malignant lesions still rests on histopathological examination [25].
The aim of this study was to assess various histopathological proliferative lesions and to know the frequency of these lesions in peritumoural area in mastectomy specimens of carcinoma breast.
The main objective of the present study was to determine the presence of any changes in periumoral area of carcinoma breast and to compare the pathological findings of tumour and adjacent area for co-existing benign proliferative lesions. The presence of different lesions within the same breast in peritumoural area is also a phenomenon that can prove the multistep progression to malignancy.
Materials and Methods
It was a prospective, cross-sectional and observational study, conducted in the histopathology section of Department of Pathology, Jawaharlal Nehru Medical College (JNMC) in Sawangi (M) Wardha, from 1st August 2017 to 31st July 2019. The study was undertaken with the approval of Institutional Ethics Committee {DMIMS(DU)/IEC/2017-18/6720}. An informed consent was taken from all the patients who were included in the study. The patients admitted in the Department of Surgery, Acharya Vinoba Bhave Rural Hospital (AVBRH) in Sawangi (M) Wardha and underwent Modified Radical Mastectomy (MRM) for carcinoma of breast were eligible to participate.
A total of 75 cases of breast carcinoma were evaluated in the present study. The sample size of 60 was calculated by using the following formula:
n=(Z a/2)2 × p × (1-p)/d2 where,
Z α/2=level of significance at 5% that is 95% confidence interval.
p=Prevalence of benign breast disease
d=Desired error of margin
n=Number of cases
Demographic data and relevant clinical details were noted from the case files and clinicopathological information was collected from requisition forms.
Inclusion Criteria:
Female patients diagnosed with invasive ductal carcinoma of breast on fine needle aspiration.
Patients who underwent modified radical mastectomy.
Patients of all age groups.
De-novo diagnosed cases of breast malignancy.
Patients not on any other treatment.
Exclusion Criteria:
All cases where only a trucut biopsy or lumpectomy or quadrantectomy had been done, as in such cases all the parameters were not available for assessment.
All cases malignancy other than infiltrating ductal carcinoma-not otherwise specified.
All cases where neoadjuvant chemotherapy is already taken by the patients.
Cases of recurrence.
Male patients excluded from the study.
The resected mastectomy specimen was fixed in 10% neutral buffered formalin. In every case standard protocol of surgical grossing of mastectomy specimen was followed. On the date of receiving mastectomy specimen, specimen was measured, grossly examined and serially sectioned at 1 cm interval from the nipple areola complex till the lateral skin margin on both ends. This step is done for fixation of the tumour mass. The recommended maximum fixation time is 72 hours.
After a detailed specimen description, it was oriented according to margins and dissection of specimen for various sections was done on the next day. Section from surgical margins, nipple and areola complex, base of tumour mass, four to five sections from the tumour mass and all the axillary group of lymph nodes were taken [26]. The peritumoural area was examined for any lesions. Even in the absence of obvious gross lesions, two or more bits were taken for histopathological examination.
According to standard protocol in histopathology division, tissue sections from representative areas were processed in the automated tissue processor to make multiple sections of 4-5 micron thickness followed by Haematoxylin and Eosin staining (H&E). All the slides prepared with H&E were reported and infiltrating ductal carcinoma was graded using the Modified Scarff Bloom Richardson grades [Table/Fig-1,2,3,4,5,6 and 7].
Section showing cystically dilated duct with apocrine metaplasia. (H&E, 10x).
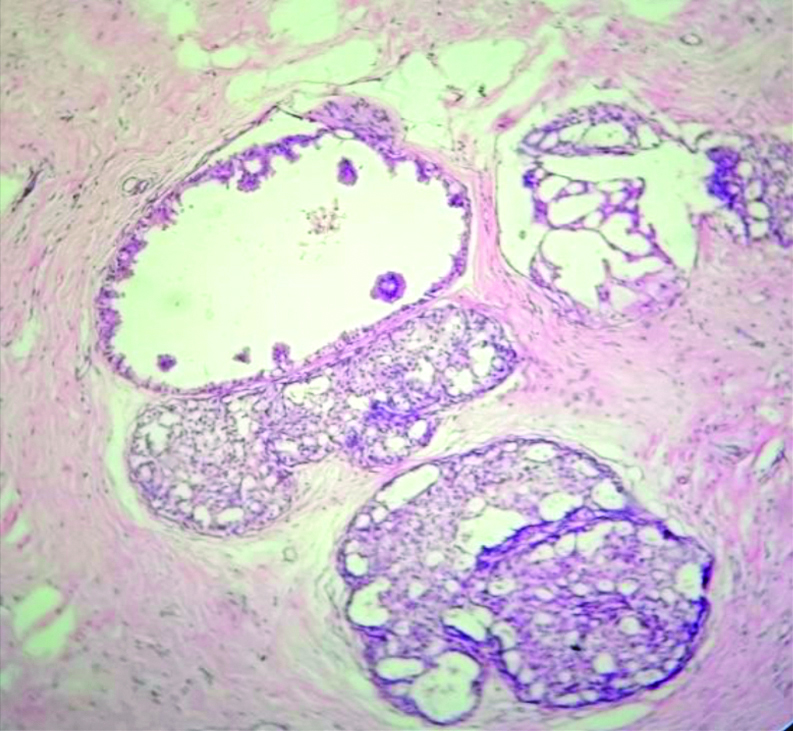
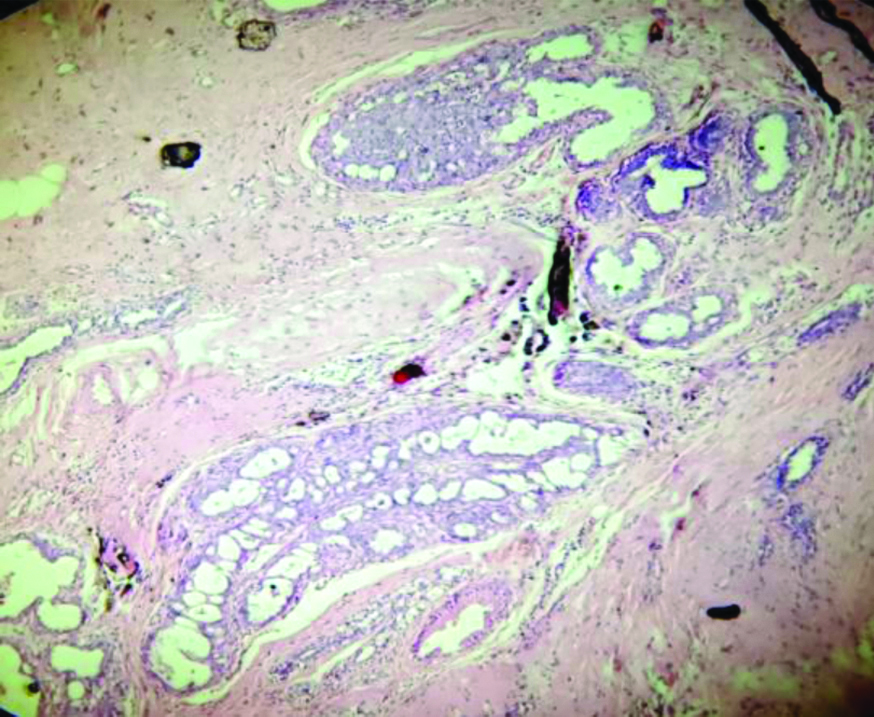
Section showing Invasive Ductal Carcinoma-Not Otherwise Specified (IDC-NOS) with cystically dilated ducts, fibrosis and haemorrhagic areas in peritumoural area. (H&E, 10x).
Section showing simple florid ductal hyperplasia. (H&E, 10x).
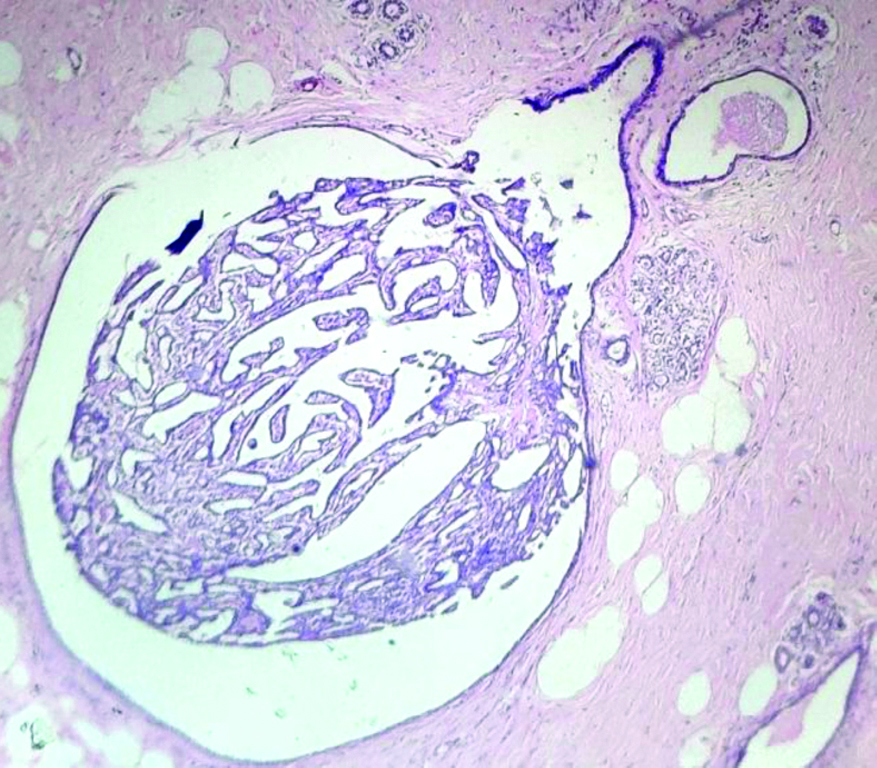
Section showing intraductal carcinoma of breast with moderate and atypical hyperplasia. (H&E, 10x).
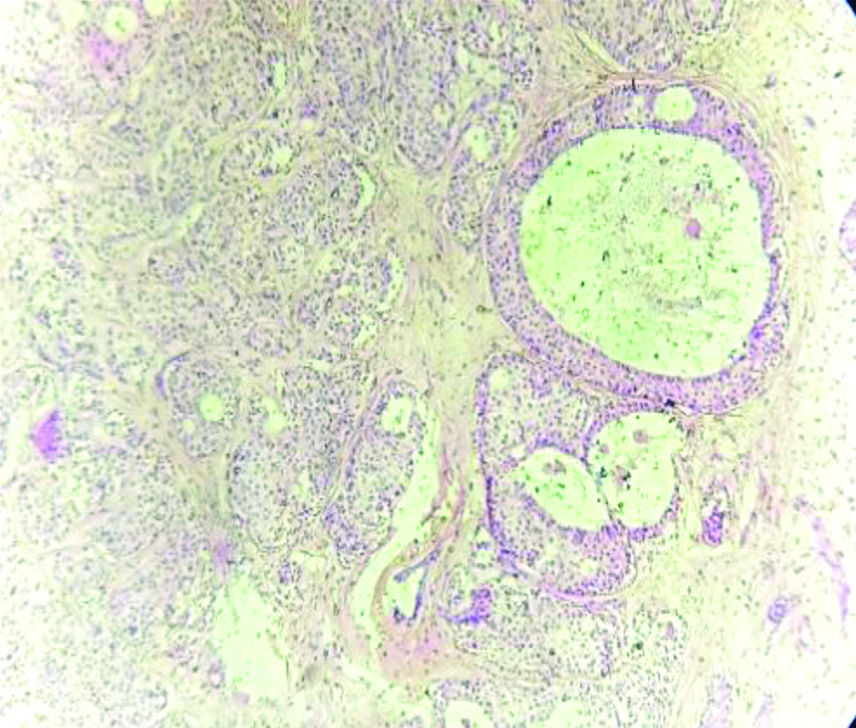
Section showing IDC-NOS with atypical ductal hyperplasia. (H&E, 10x).
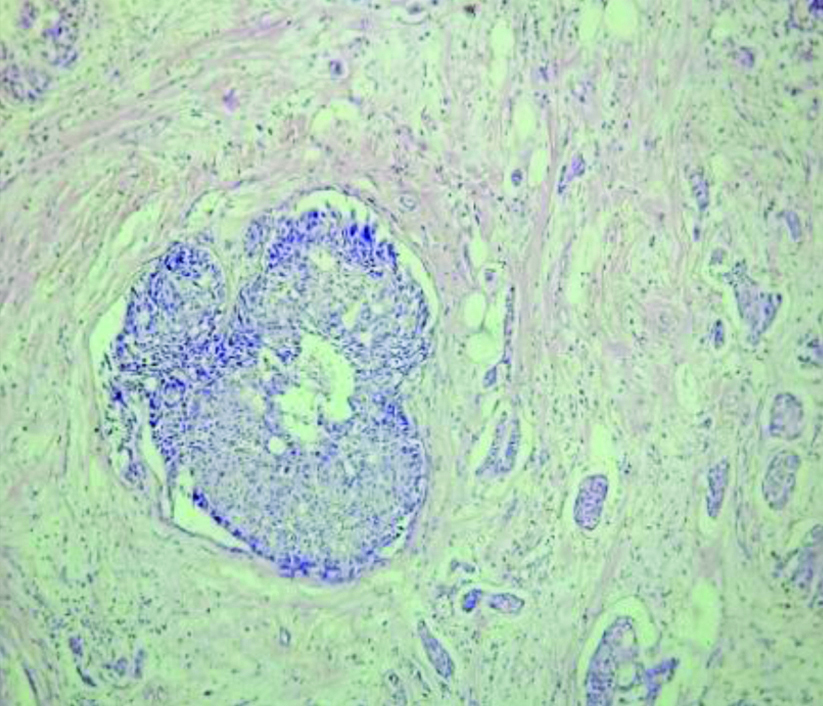
Section showing IDC-NOS with atypical ductal hyperplasia. (H&E, 10x).
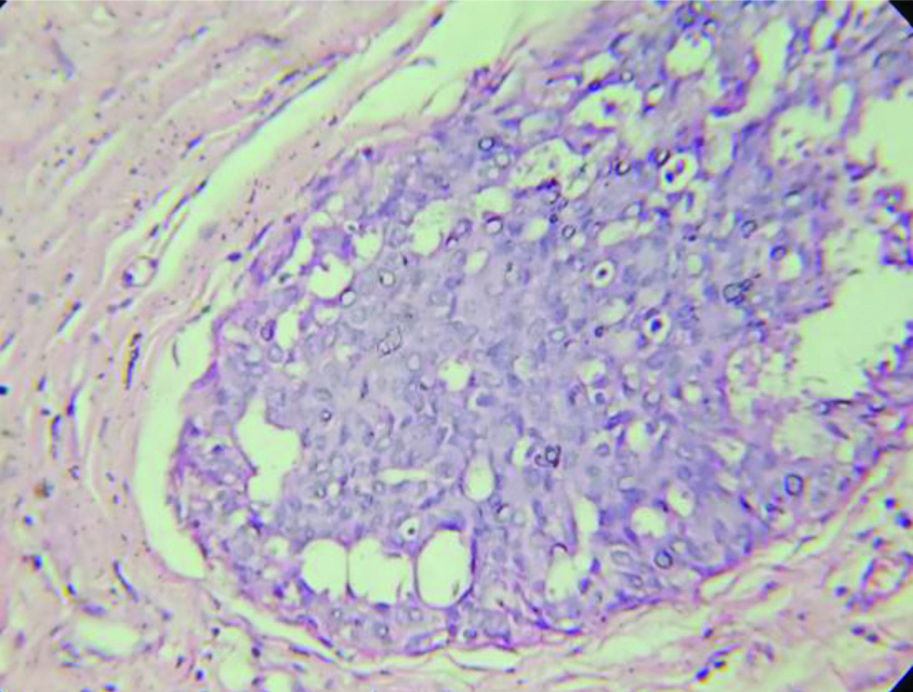
Section showing IDC-NOS with atypical ductal hyperplasia. (H&E, 40x).
In stained slides, sections of the tumour and peritumoural area were studied in detail for co-existing lesions. The lesions were classified morphologically on microscopy where tumour infiltration was not seen from the nearest point of the slides of tumour mass [27]. Based on the predominant epithelial benign lesions present in peritumoural area, the lesions were classified according to the criteria of Dupont, Page and Rogers: Non-proliferative disease, Proliferative disease without atypia and Proliferative disease with atypia [28].
Statistical Analysis
Statistical analysis was done using descriptive and inferential statistics with the chi-square test. Software used in the analysis was SPSS 24.0 version and GraphPad Prism 7.0 version. The p-value less than 0.05 were considered as level of significance.
Results
All the results of histopathological lesions in peritumoural area were noted, interpreted and recorded in tabular form.
Total 75 cases were evaluated in present study. The age range in 75 cases of breast carcinomas was 24-77 years. Mean age at diagnosis was found to be 53.33±13.26 years. In this study, maximum incidence of breast cancer was found in fifth decade followed by seventh and sixth decade. It was also found that 48% of women (36 cases) were in age group of 25-50 years. It was found that 32 cases (42.67%) belonged to postmenopausal age group.
In present study, the tumour was most frequently located in upper outer quadrant 29.33% (22 cases) followed by multiple quadrant and central area. Out of 75 cases, 66.67% had tumour mass measuring 2-5 cm (50 cases), 28% had mass of more than 5 cm (21 cases) and 5.33% had mass of less than 2 cm (4 cases). Thus, maximum cases belonged to T2 group. Grade II invasive breast carcinoma (68%) was the most common (51 cases) according to the Nottingham modification of the Bloom Richardson Grading System [29]. A 45.33% had no metastasis to lymph nodes (34 cases) while 54.67% had positive infiltration by malignant cells in lymph nodes (41 cases) [Table/Fig-8].
Distribution of cases according to age at diagnosis, mean age, menopausal status, tumour size, grade of tumour based on Bloom Richardson (BR) score, lymph node metastasis with benign breast disease.
| Characteristics | Non-proliferative disease (N=29) | Proliferative disease without atypia (N=8) | Proliferative disease with atypia (N=33) | No significant morphological change (N=5) | Total no. cases (N=75) |
|---|
| Percentage of total | 38.67 | 10.66 | 44 | 6.67 | 100 |
| Age at diagnosis (Years)- N (%) |
| <30 | 0 (0) | 0 (0) | 1 (1.33) | 1 (1.33) | 2 (2.66) |
| 30-39 | 4 (5.33) | 2 (2.67) | 1 (1.33) | 0 (0) | 7 (9.33) |
| 40-49 | 8 (10.67) | 3 (4) | 11 (14.67) | 1 (1.33) | 23(30.67) |
| 50-59 | 4 (5.33) | 1 (1.33) | 8 (10.67) | 1 (1.33) | 14(18.67) |
| 60-69 | 11 (14.67) | 2 (2.67) | 7 (9.33) | 1 (1.33) | 21(28) |
| ≥70 | 2 (2.67) | 0 (0) | 5 (6.67) | 1 (1.33) | 8 (10.67) |
| Mean age | 54.65±12.35 | 46.87±12.54 | 53.93±13.31 | 52±18.57 | 53.33±13.26 |
| Menopausal status (Years)- N (%) |
| Premenopausal (<45) | 6 (8) | 5 (6.67) | 7 (9.33) | 1 (1.33) | 19 (25.33) |
| Perimenopausal (45-55) | 9 (12) | 1 (1.33) | 12 (16) | 2 (2.67) | 24 (32) |
| Postmenopausal (>55) | 14 (18.67) | 2 (2.67) | 14 (18.67) | 2 (2.67) | 32 (42.67) |
| Tumour size (%) |
| <2 cm | 4 (5.33) | 0 (0) | 0 (0) | 0 (0) | 4 (5.33) |
| 2-5 cm | 19 (25.33) | 5 (6.67) | 24 (32) | 2 (2.67) | 50 (66.67) |
| >5 cm | 6 (8) | 3 (4) | 9 (12) | 3 (4) | 21 (28) |
| Grade of tumour based on BR score (%) |
| Grade I | 7 (9.33) | 0 (0) | 5 (6.67) | 0 (0) | 12 (16) |
| Grade II | 18 (24) | 7 (9.33) | 21 (28) | 5 (6.67) | 51 (68) |
| Grade III | 4 (5.33) | 1 (1.33) | 7 (9.33) | 0 (0) | 12 (16) |
| Lymph node metastasis (%) |
| Negative | 15 (20) | 2 (2.67) | 16 (21.33) | 1 (1.33) | 34 (45.33) |
| Positive | 14 (18.67) | 6 (8) | 17 (22.67) | 4 (5.33) | 41 (54.67) |
Chi-square test illustrated a statistically significant association of p=0.001 between benign breast disease and other variables (Menopausal status, Tumour size, Grade of tumour and Lymph node metastasis) [Table/Fig-9,10,11 and 12].
Association between menopausal status and benign breast disease.
| Menopausal status (Years)- No. of women (%) | Total no. cases (N=75) | Non-proliferative disease (N=29) | Proliferative disease without atypia (N=8) | Proliferative disease with atypia (N=33) | No significant morphological change (N=5) | χ2-value |
|---|
| Premenopausal (<45 y) | 19 (25.33) | 6 (20.70) | 5 (62.5) | 7 (21.21) | 1 (20) | 66.23 p=0.001, S |
| Perimenopausal (45-55 y) | 24 (32) | 9 (31.03) | 1 (12.5) | 12 (36.37) | 2 (40) |
| Postmenopausal (>55 y) | 32 (42.67) | 14 (48.27) | 2 (25) | 14 (42.42) | 2 (40) |
*p=0.001, S: Significant (chi-square test)
Association between tumour size and benign breast disease.
| Tumour size (%) | Total no. cases (N=75) | Non-proliferative (N=29) | Proliferative disease without atypia (N=8) | Proliferative disease with atypia (N=33) | No significant morphological change (N=5) | χ2-value |
|---|
| <2 cm | 4 (5.33) | 4 (13.80) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 76.36 p=0.001, S |
| 2-5 cm | 50 (66.67) | 19 (65.51) | 5 (62.5) | 24 (72.73) | 2 (40) |
| >5 cm | 21 (28) | 6 (20.69) | 3 (37.5) | 9 (27.27) | 3 (60) |
*p=0.001, S: Significant (chi-square test)
Association between the grade of tumour and benign breast disease.
| Grade of tumour based on BR score (%) | Total no. cases (N=75) | Non-proliferative (N=29) | Proliferative disease without atypia (N=8) | Proliferative disease with atypia (N=33) | No significant morphological change (N=5) | χ2-value |
|---|
| Grade I | 12 (16) | 7 (24.14) | 0 (0.0) | 5 (15.5) | 0 (0.0) | 75.81 p=0.001, S |
| Grade II | 51 (68) | 18 (62.06) | 7 (87.5) | 21 (63.64) | 5 (100) |
| Grade III | 12 (16) | 4 (13.79) | 1 (12.5) | 7 (21.21) | 0 (0.0) |
*p=0.001, S: Significant (chi-square test)
Association between lymph node metastasis and benign breast disease.
| Lymph node metastasis (%) | Total no. cases (N=75) | Non-proliferative (N=29) | Proliferative disease without atypia (N=8) | Proliferative disease with atypia (N=33) | No significant morphological change (N=5) | χ2-value |
|---|
| Negative | 34 (45.33) | 15 (51.72) | 2 (25) | 16 (48.48) | 1 (20) | 33.61 p=0.001, S |
| Positive | 41 (54.67) | 14 (48.28) | 6 (75) | 17 (51.52) | 4 (80) |
*p=0.001, S: Significant (chi-square test)
Discussion
Few proliferative lesions actually advance through all of the steps of initiation, progression and transformation to become invasive carcinomas, and it is likely that most of the lesions never progress beyond early stages of initiation. Hence, the identification of benign proliferative as well as involved premalignant lesions is very important in preventing progression to development of breast cancer [30]. Thus, an attempt was made to ascertain the association between these changes in peritumoural area and the primary tumour of carcinoma breast after mastectomy. The frequency of distribution of histological types of benign changes in peritumoural area noted by different authors in comparison with that of present study.
Age range: In present study, the age range and mean age of diagnosis in breast carcinomas is in concordance with Minami Y et al., Kotsopoulos J et al., Mudholkar VG et al., Shashikala R and Ravindra S and Sharma K et al., with the age shift of breast carcinomas from the age 60-70 years to 30-40 years of age [21,31-34]. Kern WH and Brooks RN [35] observed the age range from 30 to 90 years, with an average age of 61.7 years [35]. Mudholkar VG et al., also observed the age range from 27-80 years with a mean age at diagnosis of 53.39 years [32]. The maximum number of cases was found to be in the fifth decade (26.5%) which is in concordance with this study. Hoogerbrugge-van der Linden N et al., also observed that many women at high risk of hereditary breast cancer develop high-risk histopathologic lesions, especially after the age of 40 years [36]. Kotsopoulos J et al., also observed the age range was of 30 and 55 years [31]. The mean age was 54.5 for invasive cases. Shashikala R and Ravindra S, also observed the age range of the patients from 31-80 years [33]. A 65% of cases were seen in the age group of 41-60 years. A 51% of women with breast carcinoma were in the age group of 31-50 years.
Most of the patients with proliferative disease with atypia were in postmenopausal age group which is in concordance with Bayraktar S et al., [37]. Hartmann LC et al., proposed that the presence of atypia in women under 45 years of age conveyed the twice the risk observed among the women over 55 years of age which might relate to menopausal status [38]. London SJ et al., showed the higher risk of breast cancer among premenopausal woman with atypical hyperplasia which is in disconcordance with the present study [24].
The lump was most frequently located in upper outer quadrant which is in concordance with Mudholkar VG et al., and Sharma K et al., [32,34]. Most of the cases were in T2 category (tumour size 2-5 cm) which is in concordance with Baveja P and Singh B [39]. In the present study, maximum cases were in grade II category (68%). Mudholkar VG et al., (58.49%) and Sharma K et al., (46.91%) also found maximum cases in grade II category which is similar to our study [32,34] while Baveja P and Singh B found maximum cases in grade III category [39].
In the present study, the most common lesion found in peritumoural area of breast neoplasm was proliferative changes with atypia (44%) seen in 33 cases. Tellem M et al., also found that the incidence of atypical hyperplasia to be 6 to 7 times greater than in excisional biopsies in adjacent area of malignancy which in concordance with this study [40]. Baveja P and Singh B showed the intraductal epithelial proliferations in the form of hyperplasia of usual type (50%) (mild or moderate or florid), and ductal carcinoma in-situ adjacent to malignancy which is similar to present study [39]. Sathyalakshmi R et al., showed that predominant lesion found adjacent to malignancy was usual ductal hyperplasia (60%) which is in concordance with present study [27]. Mudholkar VG et al., Shashikala R and Ravindra S, Sharma K et al., & Kern WH and Brooks RN, found fibrocystic changes as the most common lesion in adjacent breast malignancy (77.05%), (38%), (34.78%), and (71%), respectively which in disconcordance with the present study [32-35].
In this study, it was also found that fibrocystic changes and atypical ductal hyperplasia contribute altogether 82.33%. Fibrocystic changes in combination with atypical ductal hyperplasia have a strong synergistic effect in development of breast cancer. These findings are in concordance with those observed by Dupont WD et al., [23].
Thus, this study tried to substantiate that these peritumoural area changes could be the premalignant lesion in these cases.
Limitation(s)
Firstly in the study, tumour heterogeneity and improper tissue sampling can lead to under diagnosis of pathological lesions in peritumoural area as it can be missed during grossing. Secondly, the follow-up studies of histomorphological alterations in the peritumoural tissue were not in plenty in published literature. Therefore, one of the limiting factor for the present study was the follow-up of such cases.
Conclusion(s)
Proliferative lesions in the breast are recognised as one of the risk factors in developing breast carcinoma and it carries an increased risk especially in presence of atypia. The present study concludes that the most predominant histological lesion in peritumoural area of carcinoma breast is proliferative changes with atypia, followed by fibrocystic changes. The study also concludes the association between the menopausal status and the histopathological proliferative lesions in peritumoural area of carcinoma breast. Proliferative lesions are associated with premenopausal age group while non-proliferative lesions are associated with postmenopausal age group. This finding can be one of the causes that explains age shift of breast carcinomas from older age group to younger group of age. Thus, histopathological examination of peritumoural area in mastectomy specimens is a simple and valuable method for stratifying the risk of carcinoma in contralateral breast also.
It is recommended that study of all four quadrants can detect high risk intraductal proliferations. The proliferative markers (e.g., Ki-67) can also be used in peritumoural area to assess the risk of proliferation and transforming into malignancy.
*p=0.001, S: Significant (chi-square test)
*p=0.001, S: Significant (chi-square test)
*p=0.001, S: Significant (chi-square test)
*p=0.001, S: Significant (chi-square test)