Carcinoma of the urinary bladder is one of the most common malignancies around the world. It ranks seventh among cancers in men while 17th in females [1]. In 2012, bladder carcinoma accounts for an estimated 0.43 million new cases with a strong male predominance. Less developed regions of the world account for 60% of all bladder cancer cases and half of all bladder cancer related deaths [2,3]. Urothelial carcinoma accounted for 0.16 million cancer related deaths in 2012 [3]. The incidence of urothelial carcinoma has increased to 0.54 million in 2018 and accounted for approximately 0.2 million deaths worldwide [4]. In India, carcinomas of urinary bladder were reported as the fourth most common carcinoma affecting males. Urinary tract cancers account for 4.89% of all cancers reported (6.96% in males and 1.89% in females); urinary bladder cancers formed 5.31% of all cancer cases in men while only 0.95% in women [5].
Screening for malignancies of the urinary tract is most commonly done using voided urine cytology. It is a non-invasive test that aids in detection as well as surveillance of atypical or malignant urothelial cells [6-9]. Urine cytology has shown to be highly sensitive (94.5%) for detection of high grade urothelial tumours [10].
Urine analysis is one of the most common tests conducted in a clinical laboratory. Flow cytometry based analysers can detect particles in urine like Red Blood Cell (RBC), White Blood Cells (WBC), Small Round Cells (SRC), Epithelial Cells (EC), Yeast Like Cells (YLC) and crystals [11]. The test is done for almost all the patients attending urology clinic and can be used as a screening test to detect early urothelial carcinoma.
There is a paucity of literature on the topic of the study of urine cytology. To best of our knowledge, no study has been conducted which evaluates the flow cytometry based parameters with voided urine cytology to detect urothelial carcinomas. The objective of the study was to establish benchmarks using parameters from UX2000 urine analyser which could be used as an advantage for isolating cases with suspicious atypical cells or with malignant urothelial cells during routine urine examination.
Materials and Methods
The prospective study was conducted in the Department of Pathology, Rajiv Gandhi Super Speciality Hospital, Tahirpur, Delhi, India. All the samples received for urine cytology for malignant cells from July 2017 to June 2018 were included in the study. All the tests were performed on the samples left after the routine procedure. No fresh sample was requested for the current study. Hence, no ethical clearance was obtained for this study. An informed consent was obtained at the time of sample collection stating that the remained of sample and test data in de-identified condition may be used for quality improvement, research studies, presentations and publications. The samples were collected in a sterile container. Samples with inadequately filled requisition forms, samples with an inadequate amount, samples collected two hours ago or samples collected outside the hospital were excluded from the study.
Each sample underwent flowcytometric examination using the Sysmex UX 2000 (Sysmex, Japan), a fully automated urine analyser. The UX 2000 aspirates 1.2 mL for Flowcytometric (FCM) analysis. The aspirated sample is segregated in two channels, namely WBC and bacterial channels, and is stained with polymethine dye. The stained sample passes through a fluidic system which arranges the cells in a linear file. Thereafter, a laser beam strikes each particle individually and produces forward scattered light signal, laterally scattered light signal and lateral fluorescent light signal which is read by arrays of detectors and electrical signals are generated which are represented as scattergrams. The particles in urine are segregated into RBC, WBC, EC, crystals, SRC, YLC and bacteria using a classification algorithm [11,12].
All the samples were also centrifuged at 1000 rpm for 10 minutes, smears prepared from the sediment were stained with Giemsa stain (Merck, Mumbai, India) and Papanicolaou stain (Biolab Diagnostics Pvt., Ltd., MS, India). Each slide was analysed by two cytopathologists before coming to a final diagnosis. The samples were reported as per the Paris system of reporting urinary cytology [13].
Statistical Analysis
Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), Odd’s ratio and Area Under the Curve (AUC) were calculated using Microsoft Excel 2010 (Microsoft Corporation, Washington, USA) along with SPSS statistical program, Version 24 (IBM, Chicago, USA).
Results
Total of 160 patients were included in the study. Majority of the patients registered in the study were males, with male to female ratio 5.67:1. Male and female patients registered in the study were 136 (85%) and 24 (15%), respectively. The age of the study group ranged from 20 years to 85-year-old.
Out of 160 cases, 35 (21.9%) cases turned out to be positive for malignant cells, while 125 (78.1%) were negative. Of the 35 positive cases, 31 (88.6%) were men and 4 (11.4%) were women. The mean age for men showing positive results was 59.8±35.2 years, while the mean age for men with negative results was 47.9±26.9 years. Similarly, the mean age for women showing positive for malignant cells was 62.3±22.6 years, while for negative was 55.3±20.4 years. Of the 35 positive cases, only 22 cases were confirmed histopathologically. Rest were lost to follow-up for histopathological examination. The histopathological diagnosis of 21 cases matched with the cytological report. Just a single case didn’t correlate with the cytological diagnosis.
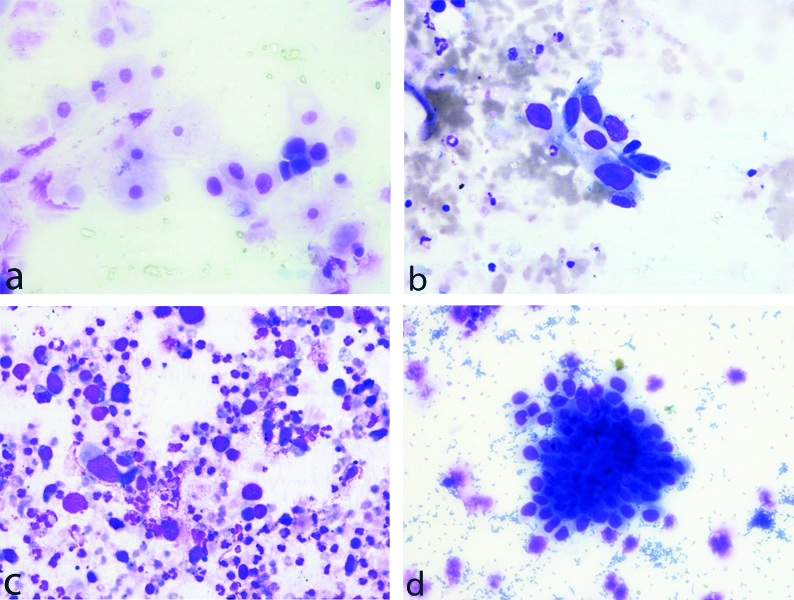
The positive specimens were reported as per the Paris System of reporting urine cytology [13]. Of the positive 35 samples, 27 (77.1%) cases were reported as category 5-High Grade Urothelial Carcinoma (HGUC), 3 (8.6%) as Category 6-Low Grade Urothelial Carcinoma (LGUC), 1 (2.9%) as Category 4-Suspicious of HGUC and remaining 4 (10.5%) were reported as Category 7-Other: primary and secondary malignancies and miscellaneous lesions [Table/Fig-1,2a-d].
Case distribution as per Paris system of reporting urine cytology.
| Paris system for reporting urine cytology | No. of cases |
|---|
| Category 1 | Nondiagnostic/unsatisfactory | 7 |
| Category 2 | Negative for High Grade Urothelial Carcinoma (NHGUC) | 112 |
| Category 3 | Atypical Urothelial Cells (AUC) | 6 |
| Category 4 | Suspicious for High-Grade Urothelial Carcinoma (SHGUC) | 1 |
| Category 5 | High-Grade Urothelial Carcinoma (HGUC) | 27 |
| Category 6 | Low-Grade Urothelial Neoplasm (LGUN) | 3 |
| Category 7 | Other: primary and secondary malignancies and miscellaneous lesions | 4 |
a: Category 3-Atypical Urothelial Cells (AUC). b: Category 4-Suspicious for High-Grade Urothelial Carcinoma (SHGUC).c: Category 5-High Grade Urothelial Carcinoma (HGUC). d: Category 6-Low Grade Urothelial Neoplasm (LGUN).
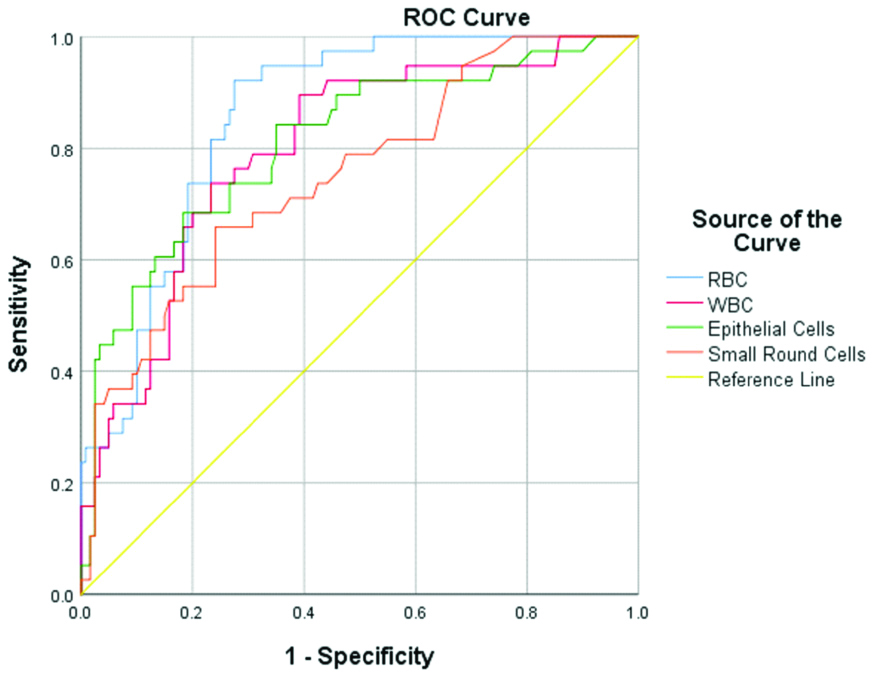
Multiple parameters, namely RBC, WBC, EC, SRC, YLC and crystals, were evaluated with results of urine cytology for malignant cells. Receiver Operating Curves (ROC) were plotted for all the parameters. Of all the parameters evaluated, RBC, EC, WBC and SRC showed statistical significance. AUC of RBC was 0.857 (95% CI, 0.80-0.92; p<0.0001), of EC was 0.809 (95% CI, 0.73-0.89; p<0.0001), of WBC was 0.798 (95% CI, 0.72-0.88; p<0.0001) and of SRC was 0.750 (95% CI, 0.66-0.84; p<0.0001) [Table/Fig-3,4]. Using ROC, cut-offs were established for all the statistically significant parameters. Only RBC, at cut-off of 52.9 cells/μL, showed high sensitivity (92.1%), specificity (72.5%), NPV (96.7%) and Odd’s Ratio of 31.5, whereas, the PPV was 51.5%. Rest of the studied parameters showed high NPV only.
Analysis of multiple parameters.
| AUC | 95% CI | Cut-off | Sensitivity | Specificity | PPV | NPV | Odd’s ratio |
|---|
| RBC | 0.857 | 0.80-0.92 | 52.9 | 92.1 | 72.5 | 51.5 | 96.7 | 31.5 |
| WBC | 0.798 | 0.72-0.88 | 44.5 | 76.4 | 72.5 | 40.6 | 90.6 | 8.5 |
| EC | 0.809 | 0.73-0.89 | 11.5 | 76.4 | 73.3 | 46.7 | 89.8 | 8.5 |
| SRC | 0.750 | 0.66-0.84 | 4.4 | 68.4 | 69.2 | 41.3 | 87.4 | 5.0 |
AUC: Area under the curve; CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value; RBC: Red blood cell; WBC: White blood cells; SBC: Small round cells; EC: Epithelial cells
Receiver operating curves (ROC) of WBC, RBC, EC and SRC.
Discussion
Urinary bladder carcinomas are among the most common cancers. It accounts for 3% of new cancers worldwide and shows an increasing trend across the globe [14]. In 2018, 549,393 new cases of urinary bladder carcinoma cases were recorded worldwide and accounted for 199,922 bladder carcinoma related deaths. The highest incidence was reported in Southern Europe while the lowest was recorded in East Africa. Higher incidence and mortality was noted in more developed world as compared to lesser developed countries [4]. The age standardised incidence is 9 per 100,000 for men and 2 per 100,000 women worldwide. As per European Union, the same is 27 per 100,000 among men and 6 per 100,000 among women [1]. In India, genitourinary malignancies formed 17.48% of all cancers among men with bladder carcinomas accounting for 30.4% of all genitourinary malignancies [15].
Sex ratio of urothelial carcinomas varies around the world with majority showing a male preponderance. According to a study conducted in Lucknow, India by Gupta P et al., the mean age was reported as 60.2±4.4 years with male to female ratio was 8.6:1 [16]. Another Indian paper showed that marked male preponderance in Indian population (8.9:1) [15]. The sex ratio for urothelial carcinomas varies around the world. A study conducted at Marburg University Hospital, German showed a sex ratio of 3.6:1 wherein 479 patients were diagnosed with urothelial carcinomas [17]. A different observation was noted in a study conducted in Taiwan which showed a female preponderance where 239 female patients were diagnosed with urothelial carcinomas as compared with 182 males [18]. The sex ratio in the present study correlates with the Indian data with male to female ratio is 7.8:1. It was also observed that the age of patients with malignancy was significantly higher than as seen in negative cases. The youngest positive case in the study was 35 years as compared to 20 years for non-malignant cases.
The risk factors for bladder carcinoma include tobacco smoking, occupational exposure to paints, dyes, metal and petroleum products, exposure to ionising radiation, Schistosomiasis and genetic predisposition [1]. Quantity and duration of tobacco smoking showed the most significant association with bladder carcinoma [15].
Of the many parameters assessed by UX2000 urine analyser, the study evaluated four parameters for the current study in correlation with urothelial malignancies; namely RBC, WBC, EC and SRC. Out of these RBC was found to be most significant and had the highest AUC of 0.857.
Haematuria is one of the common findings on routine urine examination [19]. Carcinomas of the urinary tract most commonly present with painless haematuria [14-16]. Significant microscopic haematuria is defined by the American Urological Association as more than 2 RBCs/hpf on two microscopic urinalysis without recent menses, exercise, sexual activity or instrumentation [20]. Causes of microscopic haematuria could be genitourinary malignancy, stone in the urinary tract, urinary infection, prolonged bleeding or medications like anticoagulants, aspirin, non-steroidal anti-inflammatory drugs, etc., [21]. Loo RK et al., reported that high grade haematuria (>50 RBC/hpf) was a definite risk factor with 6.36 times higher risk for genitourinary tract malignancy [22]. The mean RBC counts were 2309.3 RBC/hpf in positive cases in present study as compared to 181.3 RBC/hpf seen in the negative cases. Majority of the specimen positive for malignant cells showed high grade haematuria as compared with the negative specimens. Of all the parameters studied, only RBC counts proved to be the most sensitive, specific and with highest NPV. The other statistically significant parameters were WBC counts, EC and SRC. A mean of 2131 WBC/μL was noted in specimens positive for urothelial carcinomas as compared to 251 WBC/μL in negative cases. Elevated WBC levels in urothelial cancers could signify that urothelial carcinomas are associated with pyuria. Few studies have proven that pyuria is associated with higher pathological tumour stage, higher risk of recurrence of bladder carcinoma and a poorer prognosis [23-25]. The study have noted similar results; elevated WBC counts correlated with higher histological grade.
SRC is another parameter which was evaluated in the present study for its relation with urothelial carcinomas. SRC are generally detected in advanced renal failure and could be used as a marker for the same in diabetic as well as non-diabetic kidney diseases [26]. SRC in the urine could be used as an early indicator of diabetic renal disease [27]. SRC detected by the UX2000 was also statistically significant. SRC proved to have a high NPV whereas low sensitivity and specificity for urothelial cancers.
EC showed high NPV. All the specimens with elevated EC counts should be repeated following proper collection protocol, as it could be due to contamination. If EC were still high on a repeat sample, cytological examination should be done to exclude malignant cause.
Limitation(s)
One of the limitations noted is that this institute is one of the referral centers in Delhi and patients that are referred have high suspicion of urothelial malignancies. This leads to a skewed male to female ratio in present study as there is a higher prevalence of urothelial malignancies in men as compared to women. The study also noted that at times UX2000 urine analyser is unable to distinguish RBC from YLC and crystals. A warning flag is generated in such samples and microscopy becomes essential in distinguishing between the three parameters. This is also noted in other models of urine analysers from Sysmex (Japan) [28].
Conclusion(s)
To summarise, automated urine analysers could be used as a screening tool to detect urothelial malignancies; this would aid in isolating malignancies which would have been overlooked otherwise on a routine urine examination. Cases with an isolated increase in RBC count or in association with increased WBC count should be taken up for cytological examination to rule out urothelial malignancies; especially if the sample belongs to an elderly male patient. Early detection of malignancy could significantly alter the clinical outcome for the patient. In the end, multiple large studies are required to convert the hypothesis into practice.
AUC: Area under the curve; CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value; RBC: Red blood cell; WBC: White blood cells; SBC: Small round cells; EC: Epithelial cells