In the past the prognosis of bladder cancer depends mainly on muscle invasion; once it is found, the therapeutic approach was radical (radical cystectomy or radical radiotherapy) with poor survival outcome [4]. Hedegaard J et al., found that some cases of high grade T1 (HGT1) bladder cancer have similar behavior to that of Muscle-Invasive Bladder Cancer (MIBC) with subsequent poor prognosis [5]. Although most of cases (about 75%) diagnosed in non-muscle invasive stage [6], but about 50% of HGT1 associated with recurrence within 5 years, and 20% to 30% of the patients show progression to MIBC even following Bacillus Calmette Guerin (BCG) therapy [7].
Cancer Stem Cells (CSCs) are responsible for tumour cells heterogeneity leading to different courses of disease, growth maintenance and resistance even to combined treatment [9]. Octamer-binding transcription factor 4 (OCT4), has a vital role in keeping pluripotency of embryonic stem cells and their ability for self-renewal. It is used recently as CSCs marker [10].
Epidermal Growth Factor Receptor (EGFR) is a tyrosine kinase receptor and its activation leads to stimulation of cell proliferation [11]. Cetuximab is FDA approved antibody targeting EGFR. It is used in the management of metastatic colon and head and neck cancers and it may be beneficial in bladder cancer cases that express EGFR [12]. This study was done to shed light on OCT4 and EGFR expression in Transitional Cell Carcinoma (TCC) of urinary bladder that may be used as therapeutic targets and to investigate their predictive value in these cases.
Materials and Methods
Sixty eight cases of transitional cell carcinoma of urinary bladder admitted to Zagazig university hospital from January 2010 till December 2013 were involved in this prospective cohort study which was conducted in Pathology, Urology and Clinical Oncology departments, Faculty of Medicine, Zagazig University, Egypt.
The study included only Transurethral Resection (TUR) biopsies. Cases other than transitional type, with insufficient data, beyond T3, where muscularis propria not detected and with lymph node and distant metastasis were excluded.
The study was approved by Research and Ethical Review Committee of Zagazig university hospital (Research and Ethical Review Committee number 5688). Haematoxylin and eosin stained slides and paraffin blocks were collected from the archives of departments of pathology, Zagazig Faculty of Medicine. Clinical and pathological data were retrieved from archive files of the corresponding departments. Slides of all cases were reviewed by two pathologists.
Lamina propria invasion, muscularis propria invasion and Lymphovascular Invasion (LVI) were assessed. Histologically, tumours were divided into 2 grades, namely, low and high grades as recommended by Babjuk M et al., [6]. Stage of tumours was determined according to American Joint Committee on Cancer (AJCC) Staging Manual 8th edition [13].
The time between diagnosis to death or the most recent follow-up contact represents the Overall Survival (OS). Disease Free Survival (DFS) represents the time from beginning of treatment to date of relapse or the most recent follow-up contact when patient was relapse free.
Intermediate risk patients who underwent TUR, received induction intravesical BCG for 6 weeks followed by maintenance intravesical BCG for 1 year while high risk patients who underwent TUR received induction intravesical BCG for 6 weeks followed by maintenance intravesical BCG for 3 years using Southwest Oncology Group (SWOG) regimen. Single-dose intravesical chemotherapy (gemcitabine) was administered within 24 hours of Transurethral Resection of Bladder Tumor (TURBT). Patients with muscle invasion who underwent TUR received concurrent chemoradiotherapy using weekly dose of cisplatin.
Immunohistochemistry
Streptavidin-biotin method was applied for staining with, OCT4, EGFR and CD34 (used to confirm vascular invasion) immunohistochemically.
For immunohistochemistry, sections of 4-μm thick were cut and fixed on positively charged glass slides coated with poly L-lysine (US Biological, Swampscott, Massachusetts, USA), and were incubated for 30 minutes at 65 °C. Xylene used for deparaffinisation. Rehydration was done and sections were merged into EDTA. Slides were put in the microwave for antigen retrieval. To antagonise the activity of endogenous peroxidase, hydrogen peroxide in methanol was used followed by incubation with 1% bovine serum albumin. Sections were incubated with primary antibodies, namely:
Ready-to-use mouse monoclonal anti-OCT4 antibody (309M-18, MRQ-10). Cell Marque Corp., Rocklin, California, USA. Tissue from seminoma was used as positive control.
Ready-to-use rabbit monoclonal Anti-human Epidermal Growth Factor Receptor (EGFR), (RM-2111-R7). Thermo Scientific/Lab Vision Corporation, Fermont, USA. Skin tissue was used as positive control.
Rabbit polyclonal anti-CD34 antibody (PA5-32322, 1:100). Thermo Scientific/Lab Vision Corporation, Fermont, USA. Section from angiosarcoma was used as positive control.
After washing, slides incubated with secondary anti-rabbit antibody (Abcam) then with streptavidin horseradish peroxidase complex (Abcam). 10% of Mayer’s haematoxylin was used as counterstain. Lastly, slides were dehydrated and mounted in crystal mount.
Evaluation of Immunohistochemical (IHC) Staining
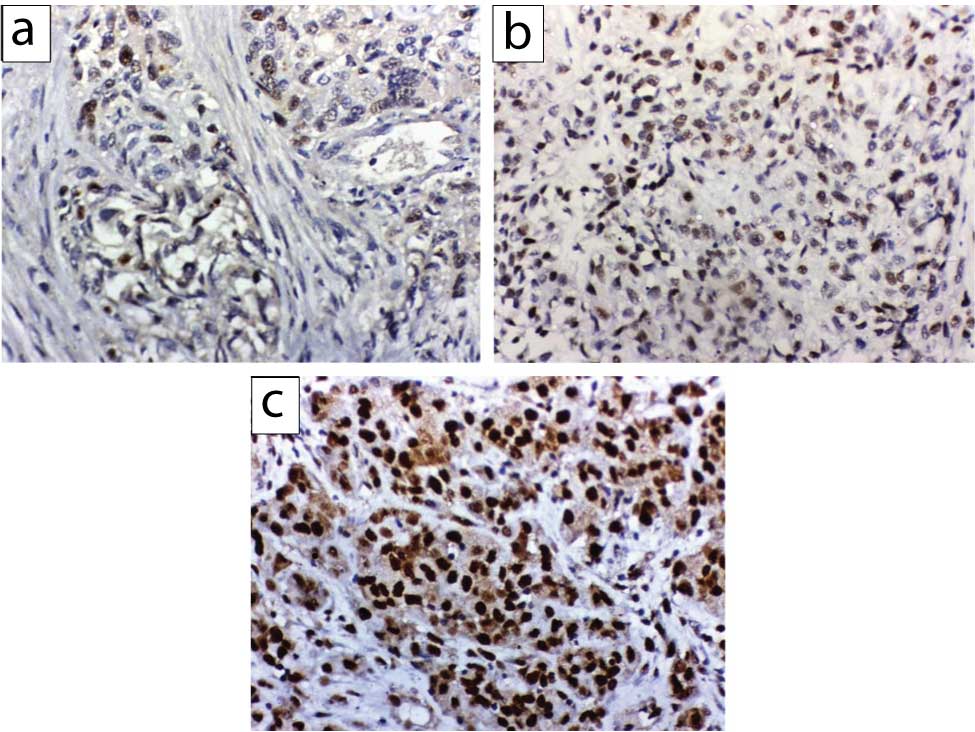
- The intensity of nuclear staining of OCT4 was categorised as follows: 0, negative; 1 weak; 2, moderate; and 3, strong [14,15]. Based on the percentage of positive cells, the degree was scored on a scale of 0 (<5%, absent), 1 (5%-25%, sporadic), 2 (25%-50%, focal) and 3 (>50%, diffuse). The final score of each staining was obtained by multiplying the two scores. The net score ranged from 0 to 9, those less than 4 was considered negative [Table/Fig-1] [14].
OCT4 mmunohistochemical expression in urothelial carcinoma; (a) weak nuclear OCT4 expression in less than 25% of tumour cells; (b) moderate nuclear OCT4 expression in 25-50% tumour cells; (c) strong nuclear OCT4 expression in more than 50% of cases (x400).
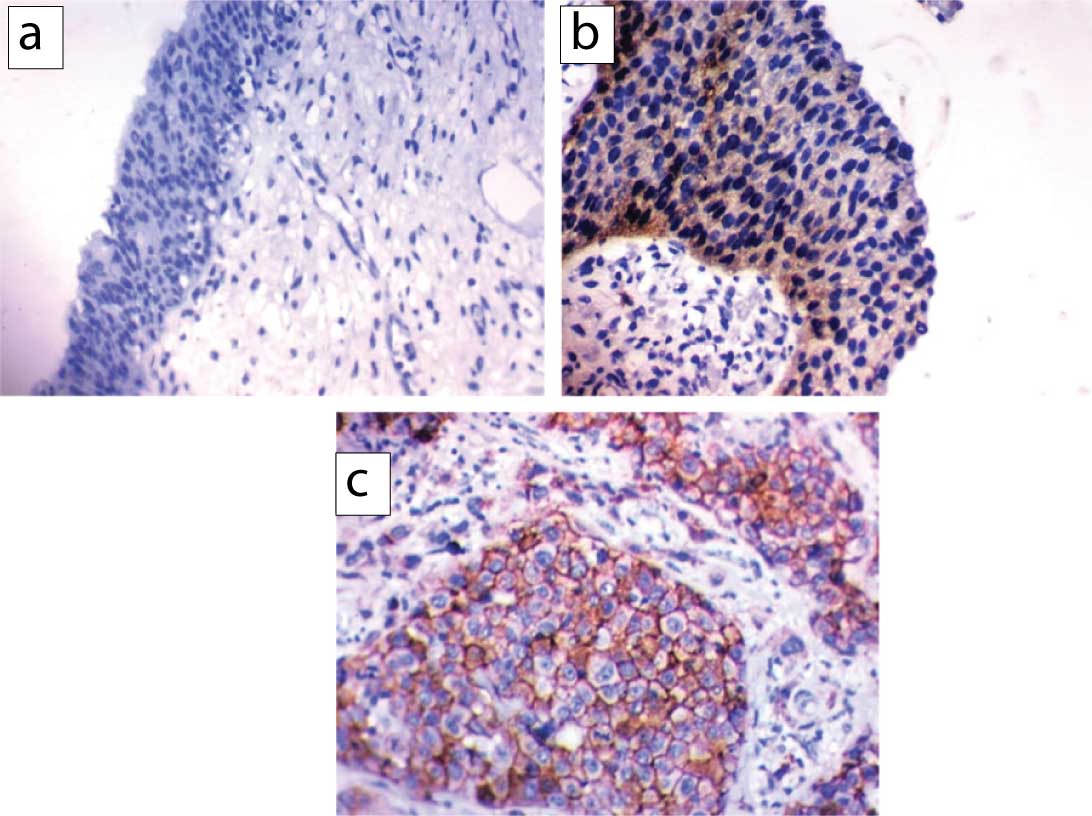
- Expression of EGFR was detected as membranous and cytoplasmic staining. The following scoring system was used to evaluate EGFR staining: score 0=no staining or unspecific staining of tumour cells or less than 10% staining, score 1=membranous weak and incomplete staining of more than 10% of tumour cells, score 2=moderate and complete membranous staining of more than 10% of tumour cells, score 3=strong and complete membranous staining of more than 10% of tumour cells. Scored1+tumours were classified as Low EGFR expression and those scored 2+or 3+were classified as High EGFR expression [Table/Fig-2] [15].
EGFR expression; a) negative expression in a case of in situ urothelial carcinomas (x200)., b) low cytoplasmic EGFR expression in non-muscle invasive TCC; and c) high EGFR expression in muscle-invasive TCC showing both membranous and cytoplasmic positivity (x400).
- CD34 staining showed circumferential staining of blood vessels.
Statistical Analysis
Normality of continuous variables was checked by Shapiro-Wilk test. For comparing two groups of non-normally distributed variables Mann Whitney U test was used. Kruskal Wallis H test was used to compare more than two groups of normally distributed variables. Percentage of categorical variables were compared using the Pearson’s Chi-square test or Fisher’s-exact test when was appropriate. Trend of change in distribution of relative frequencies between ordinal data were compared using Chi-square test. The time-to-event distributions were estimated using the method of Kaplan-Meier plot, and compared using two-sided exact log-rank test. All tests were two sided. A p-value <0.05 was considered significant. SPSS 22.0 for windows (SPSS Inc., Chicago, IL, USA) and MedCalc windows (MedCalc Software bvba 13, Ostend, Belgium) were used to perform statistics.
Results
Clinicopathological Data of Involved Cases
The study included 68 patients which were diagnosed as urothelial carcinoma. The mean age of patients was 59.18±8 years. Among them 50 (73.5%) were males. Concerning the size of tumour, 29 (42.6%) cases were >3 cm. Multifocality was detected in 24 (35.3%). A total of 36 cases (52.9%) were low grade. Lamina propria invasion was detected in 48 (70.6%) cases while vascular invasion observed in 19 (27.9%) cases and muscle invasion was detected only in 13 (19.1%) cases. According to TNM staging, 20 (29.4%) of cases were stage 0, 35 (51.5%) were Stage I, 10 (14.7%) were Stage II and 3 (4.4%) were Stage III.
OCT4 was detected in 53 (77.9%) of cases. EGFR showed high expression in 25 (36.8%) while 18 (26.5%) and 25 (36.8%) of cases revealed low and no EGFR expression respectively [Table/Fig-3].
Relation between clinicopathological features OCT4 IHC staining in 68 patients with bladder carcinoma.
| Characters | All (N=68) | Intensity of OCT4 | p-value | OCT4 | p-value |
|---|
| -ve (N=15) | Weak +ve (N=18) | Moderate +ve (N=31) | Strong +ve (N=4) | -ve (N=15) | +ve (N=53) |
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| Size (cm) |
| <3 cm | 39 | 57.4% | 11 | 28.2% | 13 | 33.3% | 13 | 33.3% | 2 | 5.1% | 0.098§ | 11 | 28.2% | 28 | 71.8% | 0.156§ |
| >3 cm | 29 | 42.6% | 4 | 13.8% | 5 | 17.2% | 18 | 62.1% | 2 | 6.9% | 4 | 13.8% | 25 | 86.2% |
| Multifocal |
| Absent | 44 | 64.7% | 8 | 18.2% | 14 | 31.8% | 21 | 47.7% | 1 | 2.3% | 0.166§ | 8 | 18.2% | 36 | 81.8% | 0.296§ |
| Present | 24 | 35.3% | 7 | 29.2% | 4 | 16.7% | 10 | 41.7% | 3 | 12.5% | 7 | 29.2% | 17 | 70.8% |
| Grade |
| Low grade | 36 | 52.9% | 13 | 36.1% | 13 | 36.1% | 9 | 25% | 1 | 2.8% | <0.001§ | 13 | 36.1% | 23 | 63.9% | 0.003§ |
| High grade | 32 | 47.1% | 2 | 6.2% | 5 | 15.6% | 22 | 68.8% | 3 | 9.4% | 2 | 6.2% | 30 | 93.8% |
| LP invasion |
| Absent | 20 | 29.4% | 8 | 40% | 6 | 30% | 6 | 30% | 0 | 0% | 0.059§ | 8 | 40% | 12 | 60% | 0.029§ |
| Present | 48 | 70.6% | 7 | 14.6% | 12 | 25% | 25 | 52.1% | 4 | 8.3% | 7 | 14.6% | 41 | 85.4% |
| Muscle invasion |
| Absent | 55 | 80.9% | 14 | 25.4% | 17 | 30.9% | 24 | 43.7% | 0 | 0% | <0.001§ | 14 | 25.5% | 41 | 74.5% | 0.719§ |
| Present | 13 | 19.1% | 1 | 7.7% | 1 | 7.7% | 7 | 53.8% | 4 | 30.8% | 1 | 7.7% | 12 | 92.4% |
| Vascular invasion |
| Absent | 49 | 72.1% | 14 | 28.6% | 16 | 32.7% | 18 | 36.7% | 1 | 2% | 0.004§ | 14 | 28.6% | 35 | 71.4% | 0.050§ |
| Present | 19 | 27.9% | 1 | 5.3% | 2 | 10.5% | 13 | 68.4% | 3 | 15.8% | 1 | 5.3% | 18 | 94.7% |
| Stage |
| Stage 0 | 20 | 29.4% | 8 | 40% | 6 | 30% | 6 | 30% | 0 | 0% | 0.001• | 8 | 40% | 12 | 60% | 0.058• |
| Stage I | 35 | 51.5% | 5 | 14.3% | 11 | 31.4% | 18 | 51.4% | 1 | 2.9% | 5 | 14.3% | 30 | 85.7% |
| Stage II | 10 | 14.7% | 2 | 20% | 1 | 10% | 5 | 50% | 2 | 20% | 2 | 20% | 8 | 80% |
| Stage III | 3 | 4.4% | 0 | 0% | 0 | 0% | 2 | 66.7% | 1 | 33.3% | 0 | 0% | 3 | 100% |
| EGFR |
| Negative | 25 | 36.8% | 13 | 52% | 8 | 32% | 4 | 16% | 0 | 0% | <0.001• | 13 | 52% | 12 | 48% | <0.001• |
| Low | 18 | 26.5% | 0 | 0% | 7 | 38.9% | 11 | 61.1% | 0 | 0% | 0 | 0% | 18 | 100% |
| High | 25 | 36.8% | 2 | 8% | 3 | 12% | 16 | 64% | 4 | 16% | 2 | 8% | 23 | 92% |
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean±SD and median (range); ‡Mann Whitney U test;†Kraskall Wallis H test; §Chi-square test; •Chi-square test for trend; p<0.05 is significant
Relation between Clinicopathological Features and OCT4 IHC Staining
Significant association detected between grade, muscle and vascular invasion, stage and intensity of OCT4 (p<0.001, <0.001, 0.004 and 0.001, respectively). Significant correlation detected between positivity of OCT4 and grade and invasion of lamina propria (p=0.003 and 0.029, respectively). A significant association between OCT4 and EGFR expression was detected (p<0.001) [Table/Fig-3].
Relation between Clinicopathological Features and EGFR IHC Staining
A highly significant association was distinguished between EGFR expression and grade, vascular invasion and stage (p<0.001 for each) and also significant association was detected with lamina propria and muscle invasion (p=0.001) [Table/Fig-4].
Relation between clinicopathological features and EGFR IHC staining in 68 patients with bladder carcinoma.
| Characteristics | All (N=68) | EGFR | p-value |
|---|
| Negative (N=25) | Low (N=18) | High (N=25) |
|---|
| No | (%) | No | (%) | No | (%) | No | (%) |
|---|
| Size (cm) |
| <3 cm | 39 | (57.4%) | 19 | (48.7%) | 12 | (30.8%) | 8 | (20.5%) | 0.005§ |
| >3 cm | 29 | (42.6%) | 6 | (20.7%) | 6 | (20.7%) | 17 | (58.6%) | |
| Grade |
| Low grade | 36 | (52.9%) | 20 | (55.6%) | 10 | (27.8%) | 6 | (16.7%) | <0.001§ |
| High grade | 32 | (47.1%) | 5 | (15.6%) | 8 | (25%) | 19 | (59.4%) | |
| LP invasion |
| Absent | 20 | (29.4%) | 14 | (70%) | 3 | (15%) | 3 | (15%) | 0.001§ |
| Present | 48 | (70.6%) | 11 | (22.9%) | 15 | (31.2%) | 22 | (45.8%) | |
| Muscle invasion |
| Absent | 55 | 80.9% | 23 | (41.8%) | 18 | (32.7%) | 14 | (25.5%) | 0.001§ |
| Present | 13 | 19.1% | 2 | (15.4%) | 0 | (0%) | 11 | (84.6%) | |
| Vascular invasion |
| Absent | 49 | (72.1%) | 24 | (49%) | 16 | (32.7%) | 9 | (18.4%) | <0.001§ |
| Present | 19 | (27.9%) | 1 | (5.3%) | 2 | (10.5%) | 16 | (84.2%) | |
| Stage |
| Stage 0 | 20 | (29.4%) | 14 | (70%) | 3 | (15%) | 3 | (15%) | <0.001• |
| Stage I | 35 | (51.5%) | 8 | (22.9%) | 15 | (42.9%) | 12 | (34.3%) |
| Stage II | 10 | (14.7%) | 3 | (30%) | 0 | (0%) | 7 | (70%) |
| Stage III | 3 | (4.4%) | 0 | (0%) | 0 | (0%) | 3 | (100%) |
Categorical variables were expressed as number(percentage), continuous variables were expressed as mean±SD and median (range); ‡Kraskall Wallis H test; §Chi-square test; •Chi-square test for trend; p<0.05 is significant
Relation between OCT4 and EGFR IHC Staining and Patient’s Outcome
Significant association was observed between OCT4 and EGFR expression as well as recurrence and disease free survival but not with mortality or overall survival [Table/Fig-5,6].
Relation between OCT4 and EGFR IHC staining and outcome in 68 patients with bladder carcinoma.
| Outcome | All (N=68) | OCT4 | p-value | EGFR | p-value |
|---|
| -ve (N=15) | +ve (N=53) | Negative (N=25) | Low (N=18) | High (N=25) |
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| Recurrence |
| Absent | 46 | 67.6% | 14 | 93.3% | 32 | 60.4% | 0.026‡ | 22 | 88% | 13 | 72.2% | 11 | 44% | 0.001‡ |
| Present | 22 | 32.4% | 1 | 6.7% | 21 | 39.6% | 3 | 12% | 5 | 27.8% | 14 | 56% |
| Disease Free Survival |
| Mean (months) (95%CI) | 29.73 months (27.21-32.24) | 35.08 months (33.34-36.82) | 28.14 months (25.18-31.30) | 0.024† | 33.99 months (31.75-36.23) | 30.57 months (26.25-34.90) | 24.89 months (19.87-29.92) | 0.002† |
| 1 year DFS | 83.8% | 100% | 79.2% | 96% | 88.9% | 68% |
| 2 year DFS | 69.8% | 92.3% | 63.6% | 87.3% | 71.4% | 51.4% |
| 3 year DFS | 65.9% | 92.3% | 58.7% | 87.3% | 71.4% | 41.1% |
| Mortality |
| Alive | 48 | 70.6% | 11 | 73.3% | 37 | 69.8% | 1.000‡ | 17 | 68% | 14 | 77.8% | 17 | 68% | 1.000‡ |
| Died | 20 | 29.4% | 4 | 26.7% | 16 | 30.2% | 8 | 32% | 4 | 22.2% | 8 | 32% |
| Overall Survival |
| Mean (months) (95%CI) | 32.29 months(30.74-33.84) | 31.60 months(27.68-35.52) | 32.49 months(30.84-34.13) | 0.913† | 32.29 months(30.74-33.84) | 33.33 months(30.85-35.82) | 31.92 months(29.20-34.64) | 0.740† |
| 1 year OS | 97.1% | 93.3% | 98.1% | 96% | 100% | 96% |
| 2 year OS | 79.4% | 73.3% | 81.1% | 76% | 83.3% | 80% |
| 3 year OS | 70.6% | 73.3% | 69.8% | 68% | 77.8% | 68% |
Continuous variables were expressed as mean (95%CI). Categorical variables were expressed as number (percentage). ‡Chi-square test; †Log rank test; p<0.05 is significant
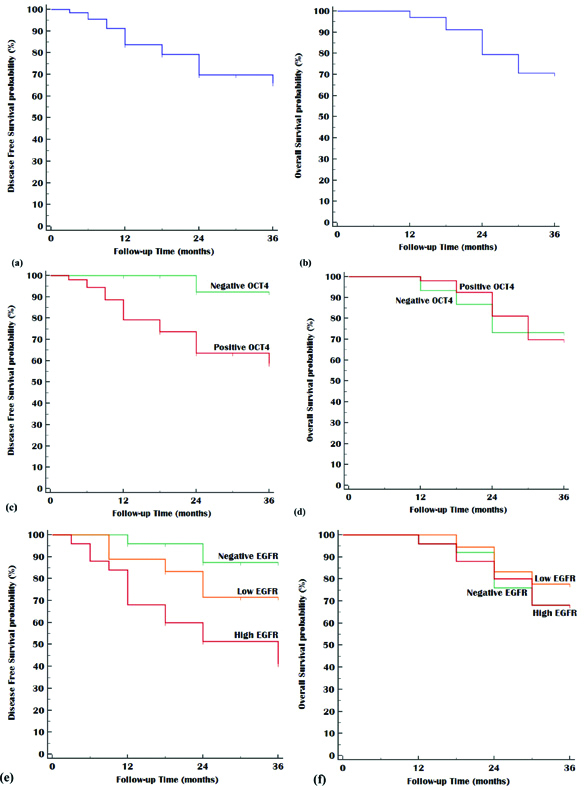
Kaplan Meier plot, Left panel: Disease Free Survival, Right panel: Overall Survival; (a&b) All studied patients, (c&d) Stratified by OCT4, and (e&f) Stratified by EGFR.
Relation between Vascular Invasion and Outcome in 68 Patients with Bladder Carcinoma
Significant association was also observed between vascular invasion and both recurrence and disease free survival but not with mortality or overall survival [Table/Fig-7].
Relation between vascular invasion and outcome in 68 patients with bladder carcinoma.
| Outcome | All (N=68) | Vascular invasion | p-value |
|---|
| No invasion (N=49) | Invasion (N=19) |
|---|
| No | (%) | No | (%) | No | (%) |
|---|
| Recurrence |
| Absent | 46 | (67.6%) | 39 | (79.6%) | 7 | (36.8%) | 0.001‡ |
| Present | 22 | (32.4%) | 10 | (20.4%) | 12 | (63.2%) |
| Disease free survival |
| Mean (months) (95%CI) | 27.44 months(29.1-33.6) | 30.12 months(27.5-32.7) | 20.51 months(15.9-25.1) | 0.002† |
| 1-year DFS | 17.6% | 12.2% | 31.6% |
| 2-year DFS | 26.5% | 18.4% | 47.4% |
| 3-year DFS | 55.9% | 69.4% | 21.1% |
| Morality |
| Alive | 48 | (70.6%) | 37 | (75.5%) | 11 | (57.9%) | 0.153‡ |
| Died | 20 | (29.4%) | 12 | (24.5%) | 8 | (42.1%) |
| Overall survival |
| Mean (months) (95%CI) | 32.29 months (33.4-35.5) | 32.81 months (30.9-34.6) | 30.94 months (27.9-33.9) | 0.130† |
| 1 year OS | 2.9% | 4.1% | 0% |
| 2-year OS | 17.6% | 12.2% | 31.6% |
| 3-year OS | 79.4% | 83.7% | 68.4% |
Continuous variables were expressed as mean (95%CI); Categorical variables were expressed as number (percentage); ‡Chi-square test; †Log rank test; p<0.05 is significant
Discussion
In spite of advances in early detection of bladder cancer in recent years, but development of drug resistance with consequent tumour recurrence is still common. One of the causes of recurrence is the presence of pluripotent stem cells that is also responsible for presence of tumours with heterogeneous cell population and different microscopic types [16]. CSCs are cancer initiating cells responsible for resistance to conventional therapy. Eradication of these cells by new treatment lines maybe valuable in overcoming the limitations of cancer treatment [17]. Octamer-binding transcription factor 4 (OCT4), is a transcription factor that plays a vital role in self-renewal of CSCs [18].
Treatment of urothelial carcinoma with chemotherapeutic agents in muscle invasive bladder cancer decreases response to EGFR target therapy [19]. That is why the authors think that identification of cases with EGFR overexpression, that may benefit from its targeted therapy before rash in use of chemotherapeutic agent, is highly valuable and may give a better response.
The current study was the first to study the association between OCT4 and EGFR expressions in bladder cancer. In the present study, the expression of OCT4 was examined in 68 cases of TCC in different stages by immunohistochemistry. Among these cases 77.9% were OCT4 positive. Sedaghat S et al., reported OCT4 expression in 93% of bladder tumour [20]. Huang P et al., [21], found it in 68% of involved bladder cancer cases while Asar A et al., [22] found it in 35.7% of cases. This discrepancy may be due to different method of evaluation as Asar A et al., divided cases according to the mean of H-score [22]. In this study, the mean of OCT4 expression was not used because it is affected by extreme values and this gives false picture about results.
In this work, OCT4 staining was detected mainly in the nucleus. Cases with strong nuclear staining showed cytoplasmic staining and this may be explained by the presence of OCT4 inactive isoform in the cytoplasm of neoplastic cells [23]. No significant relation was found between intensity of OCT4 immunostaining and the size of the tumour. Similar findings were observed by Sedaghat S et al., [20]. Although OCT4 expression was detected in all grades of TCC, significant association was present between its expression and high grade of tumour. These results were in line with finding of Sedaghat S et al., Huang P et al., Asar A et al., and Abdou AG et al., reported presence of OCT4 only in high grade tumours [20-22,24].
As regards ability of invasion by tumour cells, intensity of OCT4 immunoexpression was associated with muscularis propria and vascular invasion which agreed with finding of Sedaghat S et al., [20]. They also observed similar correlation between intensity of OCT4 and lamina propria invasion. Moreover, Asar A et al., [22] detected positive correlation between OCT4 expression and progression from non-muscle-invasive to muscle-invasive tumour.
Although most of cases with OCT4 expression in this study showed lamina propria invasion but the relation was not significant. Intensity of OCT4 expression was significantly associated with advanced stages of TCC. This was in concordance with findings of Sedaghat S et al., Jóźwicki W et al., [20,25].
These results may be explained by conclusion of Huang P et al., who reported that high expression of OCT4 correlated with the increased ability of tumour progression [21], metastasis, and recurrence in high-grade bladder tumours . Also, OCT4 positive cell represents cell that have stem cell nature. Features of stem cells include telomerase activation and evasion of apoptosis and this allows progression of tumour. These characters are also responsible for drug resistance with a high recurrence rate [18].
In line with the findings of Asar A et al., and Lu CS et al., high OCT4 expression in this work was associated with short recurrence-free intervals (p=0.026) [16,22]. Lu CS et al., reported that expression of OCT4 in recurrent tumours was significantly higher than those in primary tumours [16]. This may be attributed to the role of OCT4 in increasing survivin expression that promotes cancer cell proliferation and its action in maintenance of pluripotency [26].
According to results of the current study, OCT4 expression is associated with EGFR expression in bladder urothelial carcinoma. As far as authors know no previous work investigates their relation in bladder cancer. This association may be explained by finding of Abhold EL et al., who reported that activation of EGFR in head and neck squamous cell carcinoma cell lines induces different stem cell marker as CD44 and OCT4 [27]. Moreover, OCT4 is associated with resistance to gefitinib that targets EGFR in non-small cell lung cancers [14].
EGFR is one of tyrosine kinase receptors. It acts as receptor for different growth factors. In cancer, EGFR stimulation is important for induction of sustained cell proliferation [28]. In this study, EGFR was detected in 63.2% of involved cases of bladder cancers. In line with this work findings, Carlsson J et al., reported it in 71% of primary TCC cases [29]. Li J et al., detected it in 37.5 % of urothelial carcinoma [8], while Badawy AA et al., found EGFR expression in 86% of involved TCC cases [30].
In this work, a significant correlation was detected between EGFR and size of tumour (p=0.005). This was also observed by Hashmi AA et al., [15]. As regards grade of tumour, EGFR expression was significantly associated with higher grade. These findings are attributed to the enhancement of growth by action of EGFR on the neoplastic cells. The significant association between expression of EGFR and lamina propria, muscle and vascular invasion (p<0.001 for each) were detected. This was explained by its activation of transcription factors that have a role in induction of matrix metalloproteinases activity [31]. In line with Badawy AA et al., [30] EGFR expression was significantly associated with the stage of tumour (p<0.001). Similar to finding reported by Li C et al., EGFR overexpression is associated with tumour recurrence (p=0.001) and decrease disease free survival (p=0.002) [26]. Same results have been reported by Hashmi AA et al., [15].
The significant association between EGFR expression and progression presented by the stage of tumour and invasion of muscularis propria in addition to its correlation with recurrence explains its prognostic and predictive values.
Limitation(s)
Limitations of this study was inability to target these molecules to confirm their therapeutic effect.
Conclusion(s)
Using selective inhibitors for EGFR and OCT4 especially that were approved for EGFR in treating selected cases of urothelial carcinoma of bladder could suppress tumor growth. These markers in addition to presence of vascular invasion, have to be used in screening of high risk noninvasive group that benefits from targeted therapy.
Recommendation(s)
Further studies targeting EGFR and/or OCT4 on selected cases that express these markers as adjuvant treatment in urothelial bladder cancer is recommended.