Preeclampsia is a common complication of pregnancy, associated with increased morbidity and mortality in mother and fetus [1]. It is a transient but potentially dangerous, multisystem disorder which is characterised by high maternal systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg measured on two occasions separated by atleast 6 hours with associated proteinuria (protein excretion of ≥300 mg in 24 hours urine collection, or a dipstick of ≥1+) that develops after 20 weeks of gestation in previously normotensive women [2-4]. It may be associated with complications like visual disturbances, oliguria, eclampsia, HELLP syndrome (haemolytic anemia, elevated liver enzymes and low platelet count), pulmonary oedema and fetal growth restriction [5]. The associated predisposing factors are advanced maternal age, obesity, diabetes mellitus, oxidative stress and placental ischemia [4]. Among the biochemical parameters serum calcium, magnesium, vitamin D and thyroid hormone levels have been found to be associated with the occurrence of preeclampsia [1,3,6,7].
Despite its prevalence and severity and considerable research, the pathophysiology of preeclampsia is not fully understood and the exact cause is still unknown [1]. Multiple hypotheses have been put forward to explain its occurrence and thus preeclampsia is also named as ‘disease of theories’ [8]. The basic pathophysiological mechanism is probably a failure of the trophoblastic invasion of the spiral arteries, leading to maladaption of maternal spiral arterioles associated with an increased vascular resistance of the uterine artery and a decreased perfusion of the placenta [4,9]. The implicated vascular resistance and under-perfusion of the placenta, may lead to the release of antiangiogenic factors into the maternal circulation and alter maternal systemic endothelial function to cause hypertension and other manifestations of the disease [8]. Environmental and nutritional factors may also play a role in the aetiology of preeclampsia.
Calcium deficiency has been observed to associate with Pregnancy-Induced Hypertension (PIH) and preeclampsia [10]. Low serum calcium may lead to a decrease in vasodilating prostacyclin levels that may enhance vasoconstriction and increase blood pressure. Moreover, Hypothyroidism is also listed as one of the important causes of high blood pressure [11]. During pregnancy, there is an increased thyroid demand and the uptake of iodine, required for synthesis of thyroid hormones. Oestrogen induces a rise in Thyroxine Binding Globulin (TBG) and several thryroid stimulatory factors are released from placenta. However, in preeclampsia, there is failure of estrogen production due to placental dysfunction, which results in hypothyroidism along with fetal growth restriction [11]. Experimental studies have also suggested that in a state of hypothyroidism, release of Nitric Oxide (NO) is altered which may result in endothelial cell dysfunction and might be a pathogenic mechanism for hypothyroidism in preeclampsia [12]. Among the thyroid disorder cases during pregnancy, more of subclinical hypothyroidism cases have been observed as compared to overt hypothyroidism or hyperthyroidism [13]. All these studies underline the significance of thyroid hormones and their functions during pregnancy.
Furthermore, thyroid hormones have also been found to influence serum calcium levels in hypothyroid patients [14]. Despite evidence of alteration of thyroid profile and serum calcium levels in preeclampsia, to the best of our knowledge, this is the first study from India to report a relationship between a comparison or combination of these parameters and occurrence of preeclampsia. The present study, therefore, was conducted to analyse the serum calcium level and levels of hormones related to thyroid profile viz., free triiodothyronine (fT3), free tetraiodothyronine (fT4), and TSH and their inter-correlation and odds with the occurrence of preeclampsia. The relationship between the preeclamptic occurrence, serum TSH and total serum calcium level was also analysed. Furthermore, the status of total serum calcium levels and thyroid profile was correlated to BMI and birth weight of newborns so as to further establish the relation (if any) between these biochemical parameters and effects and outcomes of preeclampsia.
Materials and Methods
This, cross-sectional, case control study was conducted in the Department of Biochemistry, Jaipur National University Hospital affiliated to Jaipur National University Institute of Medical Sciences and Research Center (JNUIMSRC), Jaipur, a tertiary care center in Jaipur, Rajasthan for a period of one year (July 2018 to June 2019). Prior to the collection of blood samples, approval from Institutional Ethics Committee (IEC) was obtained ((JNUIMSRC/IEC/2018/47).
Study Population
The study protocol and the objectives of the study were explained to the enrolled subjects and their written informed consent was obtained. After a thorough clinical examination, a total of 170 pregnant women were selected: 80 in preeclampsia group (case group); 90 in normotensive group (control group), in the third trimester and between 18-45 years, visiting Gynecology OPD or admitted in JNUIMSRC were enrolled in the study. Equal number of primigravid and multigravid women were included in both case and control groups. The demographic details, obstetric history and relevant medical history of all the study subjects were recorded in a predesigned history form. Investigations were conducted in third trimester of pregnancy and as far as possible the controls and cases were age matched.
Inclusion and Exclusion Criteria
Case group: Pregnant women with blood pressure ≥140/90 mm of Hg, measured on two occasions 6 hours apart, along with proteinuria ≥1+ with urine dipstick (corresponds to ≥300 mg in 24 hours urine sample) were included in case group. False-positive dipstick tests were avoided by two random midstream urine specimen ≥4 hours apart.
Control group: Pregnant women without hypertension (normotensives) were selected if BP <140/90 mmHg with no increase in systolic BP upto 30 mmHg and diastolic BP upto 15 mmHg due to pregnancy and without any evidence of proteinuria.
Pregnant women with complications like diabetes mellitus, chronic hypertension, chronic renal disease, chronic liver disease, cardiovascular disease, endocrinal disorder, autoimmune disease, hyperuricaemia, genital malignancies, blood discrasias, acute infections, and those on anticonvulsant drugs were excluded from the study. Among the multigravida any history of hypertension, trophoblastic disease, placental abruption, malformed fetus, history of preeclampsia, metabolic disorders or convulsions were ruled out. Also, child bearing women with history of substance abuse or smoking were excluded.
Procedures
BP was measured using digital sphygmomanometer from the left arm in a sitting position after 5 minutes rest. An average of three readings obtained after a 5 minutes rest defined systolic and diastolic BP. Participants’ height, without shoes, was measured using wall-mounted stadiometer. Weight of participants, with minimal clothing, was measured to the nearest 0.1 kg on a portable scale. BMI (in kg/m2) of the participants was calculated by estimating the ratio between body weight (kg) and a square of height (in meters2). A regular follow-up of the participants was done till delivery, the birth weight of the newborns was measured.
Sample Collection
Blood samples were collected from the case as well as control subjects and were coded to avoid possible bias. A total of 10 mL venous blood was collected from each subject under aseptic conditions in a sterile syringe and was transferred to a clean and dry vial without anticoagulant. The needle was removed from the syringe to avoid haemolysis while transfer of the blood sample. The blood was allowed to clot spontaneously and then centrifuged at 3000 rpm for 15 minutes to extract serum. The extracted serum was collected in eppendorf tubes and was divided into two parts. One part was processed further for biochemical investigations within two hours of the collection and the other part was stored at -20°C for further investigations.
Freshly voided, random, midstream urine sample (15-20 mL) was collected on two different occasion’s atleast (4 hours apart) in clean, wide mouthed, leak proof containers for urinalysis.
Biochemical Investigations
Totalserum calcium levels were estimated by Arsenazo-III method using a fully automatic analyserby RANDOX, RX-imola, Crumlin, UK. Thyroid profile (fT3, fT4 and TSH) was estimated by enhanced chemiluminescence technique using a fully automatic analyser by Johnson and Johnson, VITROS®ECiQ, Immunodiagnostic system, Ortho Clinical Diagnostics, New Jersey, USA. Proteinuria was measured by Combur10 test® M strips by Roche and analysed by using semi-automatic Cobas u 411 urine analyser by Roche diagnostics, Germany, and the samples were graded as 1+, 2+ or 3+ for proteinuria. Reference range of thyroid profile [15] and total serum calcium level [16] during third trimester of pregnancy was taken as, fT3: 2.4-36.1 pg/mL; fT4: 0.47-5.1 ng/dL; TSH: 0.47-5.78 μIU/mL; Total serum calcium: 8.2-9.7 mg/dL.
Statistical Analysis
All the statistical analysis was performed using Microsoft Excel 2007. The non-categorical variables were subjected to the Kolmogorov-Smirnov test to analyse for their normal distribution. Except for fT4 and gestational age, all the other variables were found to be normally distributed. Z-test was performed on data to compare the mean values of variables under study. The difference in mean values of variables was considered significant at p<0.05. The odds ratio was applied to calculate the risk of preeclamptic occurrence with hypocalcaemia (total serum calcium <8.2 mg/dL) and hypothyroidism (serum TSH level >5.78 μIU/mL). Considering these conditions as categorical variables, their frequency in control and cases were estimated. Significance in their respective frequency change was analysed using the chi-square test. The correlation between serum TSH level, birth weight, and total serum calcium level were performed using Pearson’s correlation (p-value <0.05).
Results
The difference in the mean age of the two groups was statistically insignificant [Table/Fig-1]. The mean gestational age of cases and controls were 36.7±2.7 weeks and 37.6±2.1 weeks respectively, with a non-significant difference of one week. Total 61% of both the cases and controls were primigravid. Thus, the two groups of cases and controls had almost no differences regarding their average maternal age, average gestational age, or parity. The mean BMI of cases (25.29±3.9) was higher than that of controls (23.1±3.1).
Comparison of the variables between case and control group.
| Variable | Mean±standard deviation | p-value |
|---|
| Cases(n=80) | Controls(n=90) |
|---|
| Age (years) | 24.33±4.22 | 23±3.6 | >0.05 |
| Gestational age (weeks) | 36.7±2.7 | 37.6±2.1 | * |
| BMI (kg/m2) | 25.29±3.9 | 23.1±3.1 | <0.01 |
| fT3 (pg/mL) | 2.67±0.77 | 2.56±.69 | >0.05 |
| fT4 (ng/dL) | 1.153±0.67 | 1.00±0.38 | * |
| TSH (μIU/mL) | 5.07±3.34 | 3.3±1.75 | <0.01 |
| Total serum calcium (mg/dL) | 8.2±0.8 | 8.7±0.5 | <0.01 |
| Birth weight (kg) | 2.30±0.52 | 2.77±0.35 | <0.01 |
BMI: Body mass index; fT3: Free triiodothyronine; fT4: Free tetraiodothyronine; TSH: Thyroid stimulating hormone
Data represented as mean±standard deviation
*Variables not normally distributed as per Kolmogorov-Smirnov test
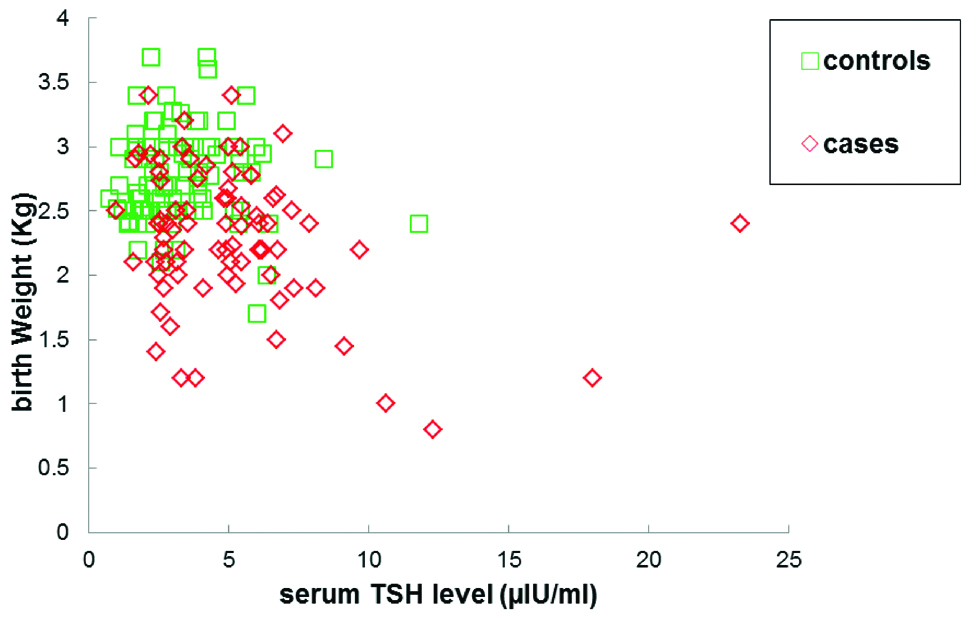
Mean TSH levels in case group were found to be significantly higher (5.07±3.34 μIU/mL) when compared with control group (3.3±1.75 μIU/mL). Significantly, lower levels of mean total serum calcium were observed in cases (8.2±0.8 mg/dL) than in controls (8.7±0.5 mg/dL). No significant differences in mean fT3 levels were observed between cases and controls. Although within the normal range, small elevations in mean fT4 levels were observed in cases (1.153±0.67 ng/mL) as compared to controls (1.00±0.38 ng/mL). Mean birth weight of the newborns of cases was significantly lower (2.30±0.52 Kg) compared to controls (2.77±0.35 Kg). Moreover, the birth weight of newborns of case subjects was found to be positively correlated with serum calcium levels (r=0.29, p=0.007) [Table/Fig-2,3] and was negatively correlated with serum TSH levels (r=-0.29, p=0.0069) [Table/Fig-3,4].
The total serum calcium levels correlated positively with birth weight and was found to be significant in preeclamptic case subjects (r=0.29, p=0.007).
No significant correlation was observed between total serum calcium levels and birth weight among the control subjects (r=0.09, p=0.38)
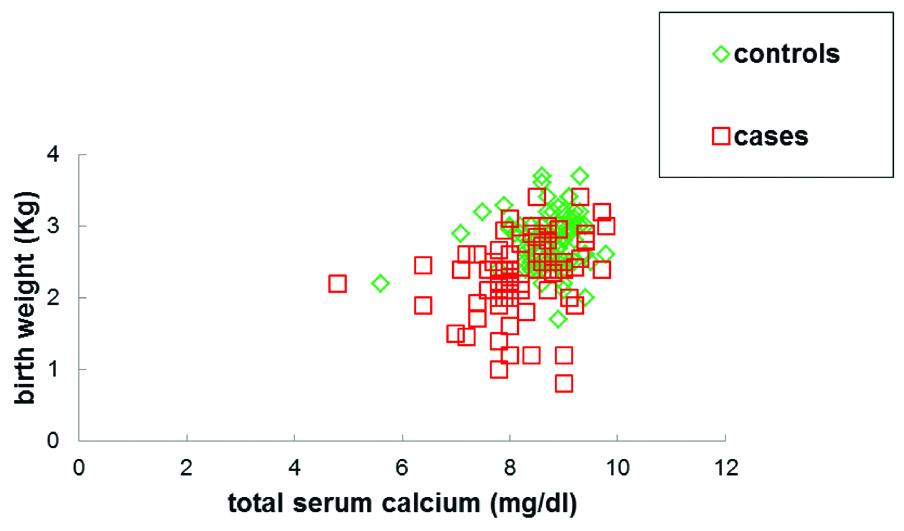
Correlation of the birth weight of newborns with total serum calcium level and TSH level.
| Birth weight | Serum TSH level | Total serum calcium level |
|---|
| Correlation coefficient (r) | p-value | Correlation coefficient (r) | p-value |
|---|
| Cases | -0.29 | 0.0069 | 0.29 | 0.007 |
| Controls | -0.029 | 0.77 | 0.09 | 0.38 |
TSH: Thyroid stimulating hormone
The serum TSH levels were observed to correlate negatively with birth weight. Observed correlation was significant in preeclamptic cases (r=-0.29, p-value=0.0069).
No significant correlation was found between serum TSH levels and birth weight in control subjects (r=-0.029, p-value=0.77)
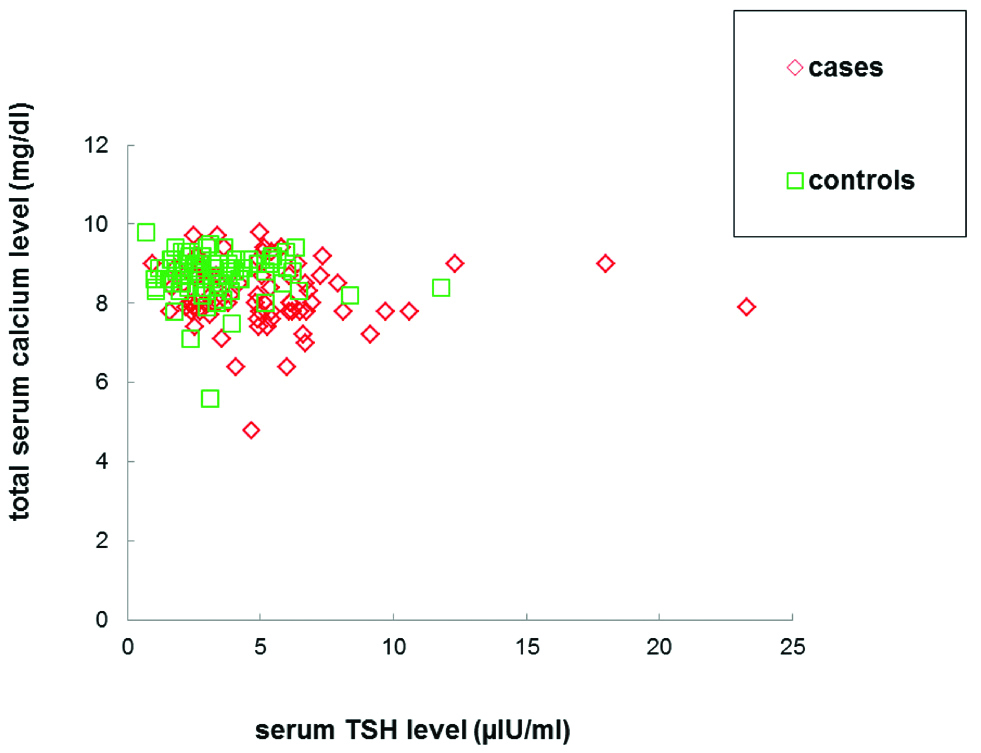
Total 51.25% of the case group subjects were hypocalcaemic (<8.2 mg/dL) as compared to 8.8% of the control subjects and with an odds ratio of 10.8, participants with total serum calcium level <8.2 mg/dL were found to have approximately 11 times higher risk for occurrence of preeclampsia. On investigating TSH levels, 32.5% of the case group subjects were subclinically hypothyroid (TSH >5.7 μIU/mL) as compared to 8.8% of the control subjects and with an odds ratio of 4.98, participants with their serum TSH levels >5.7 μIU/mL were observed to have approximately five times more risk for preeclampsia [Table/Fig-5]. In both the groups, no significant correlation was found between TSH and total serum calcium levels [Table/Fig-6]. Moreover, 18.75% of the case group subjects had subclinical hypothyroidism and hypocalcaemia simultaneously. However, interestingly, none of the control group subjects were found to have both these conditions at the same time.
Distribution of cases and controls according to thyroid status and serum calcium levels. (Cases: n=80; Controls: n=90).
| Variable | Cases, n (%) | Controls, n (%) | Odds ratio |
|---|
| TSH >5.78 μIU/mL* | 26 (32.5) | 8 (8.8) | 4.98 |
| TSH <5.78 μIU/mL | 54 (67.5) | 82 (91.1) |
| Total serum calcium <8.2 mg/dL** | 41 (51.25) | 8 (8.8) | 10.8 |
| Total serum calcium >8.2 mg/dL | 39 (48.75) | 82 (91.1) |
| TSH >5.78 μIU/mL and total serum calcium <8.2 mg/dL | 15 (18.75) | - | - |
*For comparative analysis of TSH: χ2=14.7, df=1, p<0.05,95% CI=6.726-9.831
**For comparative analysis of Total serum calcium, χ2=37, df=1, p<0.05.95% CI=7.437-7.806
TSH: Thyroid stimulating hormone; df: Degrees of freedom CI: Confidence interval
Serum TSH levels were observed to correlate negatively with total serum calcium. Observed correlation was however insignificant in both case (r=-0.04, p=0.66) and control subjects (r=-0.03, p=0.72).
Discussion
Proper nutrition prior to and throughout pregnancy has long been known for optimising the health and well being of both mother and baby [17]. Pregnancy is a period of increasing metabolic demands with changes in women’s physiology and the requirements of a growing fetus. However, pregnant women in developing nations have been reported to consume diets with lesser amounts of essential minerals and vitamins, which might be harmful not only for the mother but also for the growing fetus [18]. There are evidence that indicates a role of micronutrients supplementation in preventing some pregnancy disorders. Among these one such important micronutrient is calcium. Hypocalcaemia during pregnancy can have adverse effects on the mother as well as the growing fetus and can result in complications like preeclampsia and fetal growth disorders [10].
Various studies have evaluated serum calcium levels as potential predictors of preeclampsia [1,3,19,20] and several epidemiological studies in developing nations indicate an association between reduced calcium intake and preeclampsia. Importance of calcium in preeclampsia is further strengthened by calcium supplementation studies [21-24] where calcium supplementation (≥1 g/day) could significantly reduce the risk of preeclampsia, particularly for women with low calcium diets. Studies have shown significantly lower dietary calcium intake among the pregnant women who develop severe preeclampsia as compared to normotensive women [25]. These observations lead to hypothesis that the incidence of preeclampsia can be reduced in population of low calcium intake by calcium supplementation [23,24], which is an attractive and also a potential intervention to reduce the risk of women developing preeclampsia. In the present study, total serum calcium levels among the case group subjects were significantly lower than in control group subjects a finding, which was in tandem with previous studies [26,27]. In contrast to the present study findings, various other studies have reported that there was no significant change in serum calcium levels between preeclamptic cases and control groups [19,28]. The probable reason for such discordant findings may be due to differences in the genetic constitution and the dietary habits of the concerned populations under study. The nonexistent correlation between total serum calcium and TSH levels indicates that both these parameters probably act as independent risk factors for the occurrence of preeclampsia.
Thyroid dysfunction is frequent in pregnancy and is often associated with adverse maternal and fetal outcomes. The evidence from a previous study [29] has indicated mutual influences between thyroid function and preeclampsia. Also, there is evidence that subclinical hypothyroidism during pregnancy is related to increased risk of the occurrence of severe preeclampsia [30]. Complications in preeclampsia are attributed to decreased placental perfusion and oxidative stress with subsequent release of anti-angiogenic factors [31]. These factors may restrict the capillary flow of the thyroid hormone, thereby affecting thyroid hormone secretion and release. However, results from the present study revealed no significant difference in the mean values of fT3 levels between cases and controls, both being within the normal range. These results, while being concurrent with some studies [32], were found contradicting to the observations by some others [29,33].
Increased serum TSH levels may promote endothelial dysfunction by attenuation of endothelial nitric oxide synthase and prostacyclin expression [34]. In vitro studies with direct administration of recombinant human TSH on endothelium have shown impairment in vasodilatation, an increase in oxidative stress and an increase in production of inflammatory cytokines. This, in turn, can cause vascular endothelial dysfunction, a prominent feature of preeclampsia [35]. In the present study, the mean values of serumTSH levels in case group were significantly higher than that of control group, an observation which was in parallel to the previous studies [7,36]. Present study results in the context of serum TSH levels support the hypothesis that increased serumTSH levels should be considered as a predisposing factor for the occurrence of preeclampsia. However, this conclusion is contradicted by some studies as these did not find any significant difference in serumTSH levels among cases and controls [32,37]. These contradicting observations can be attributed to demographic variations.
A low dietary calcium intake is associated with increased BMI [38]. It is suggested that increase in dietary calcium may cause repression of intracellular Ca2+ ions in adipocytes that may result in stimulation of lipolysis and asubsequent weight loss leading to decrease in the risk of hypertension [38]. In the present study, BMI of the preeclamptic cases was observed to be higher than that in controls. The observed difference in BMI is in concurrence with previous studies [1]. However, no significant correlation of BMI with serum calcium level was observed among cases and controls. These findings suggested that additional parameters could correspond to changes in BMI and its relationship to the occurrence of preeclampsia. BMI is also considered as one of the factors influencing thyroid function in pregnancy [39]. At the molecular level, adipose tissue is known to produce leptin, cytokine, and inflammatory factors. Leptin is known to act on TRH secreting neurons, which in turn activate the synthesis of prothyrotropin releasing hormone (pro-TRH) [40]. Moreover, the expression of TSH receptors on obese people is comparatively low as compared to lean individuals [41]. A feedback stimulus, therefore, may result in a further increase in plasma TSH levels in obese individuals. In our results, the anticipated correlation or interdependence between BMI and TSH levels was found absent in cases as well as in controls. When comparing cases to controls, positive differences in mean BMI and mean TSH levels and the respective odds ratio signified association of these parameters with increased risk of preeclamptic occurrence. In context of serum TSH levels affecting BMI of the participants on route to increasing preeclamptic risk, a non-correlation between TSH levels and BMI could not explain BMI’s association with increased risk of preeclamptic occurrence through alteration in serum TSH levels.
In the present study it was observed that the birth weight of the babies of preeclamptic mothers was significantly lower than that of normotensive ones. Similar results were also observed in previous studies [1,11]. Reduction in the birth weight of babies in cases can be attributed to Intrauterine Growth Restriction (IUGR), frequently found associated with preeclampsia, possibly due to decreased placental perfusion and reduced nutritional supply to the fetus [42]. Present study results show significant positive correlation between total serum calcium levels and birth weight of babies of preeclamptic mothers may support the low calcium vasoconstriction hypertension decreased placental perfusion inefficient nutritional supply IUGR theory to explain the adverse outcome of lower birth weight of newborns in preeclampsia. Altered thyroid function is also associated with hypertension, IUGR, placental abruption, preterm birth, and low birth weight of newborns [43]. A negative correlation found between serum TSH levels of preeclamptic mothers and birth weight of their newborns further substantiates the contribution of higher TSH level in adverse outcomes of preeclampsia.
Limitation(s)
Authors realise that although the data generated from this study can serve as a template to tailor the existing guidelines but it needs to be interpreted cautiously as it may not be representative of the whole Indian scenario.
Conclusion(s)
In conclusion, results from this study emphasise on total serum calcium level (<8.2 mg/dL) as relatively better indicator and predisposing risk factor for preeclamptic occurrence than serum TSH levels (>5.7 μIU/mL). We hereby cautiously suggest that a combination of these parameters can be considered as a more reliable indicator of preeclamptic occurrence. Both these parameters probably act as independent risk factors for the occurrence of preeclampsia, a suggestion that is strengthened by a statistically insignificant correlation between serum TSH levels and serum calcium levels. Based on these results, authors suggest for the screening of thyroid profile and serum calcium levels and analysis of their combined status during pregnancy to minimise the risk of preeclamptic occurrence and to avoid adverse pregnancy outcomes.
BMI: Body mass index; fT3: Free triiodothyronine; fT4: Free tetraiodothyronine; TSH: Thyroid stimulating hormone
Data represented as mean±standard deviation
*Variables not normally distributed as per Kolmogorov-Smirnov test
TSH: Thyroid stimulating hormone
*For comparative analysis of TSH: χ2=14.7, df=1, p<0.05,95% CI=6.726-9.831
**For comparative analysis of Total serum calcium, χ2=37, df=1, p<0.05.95% CI=7.437-7.806
TSH: Thyroid stimulating hormone; df: Degrees of freedom CI: Confidence interval