Effectiveness of Prophylactic Intranasal Phenylephrine in Prevention of Nasal Congestion and Hypotension after Spinal Anaesthesia in Obstetric Patients: A Randomised Double Blinded Study
Sankar Roy1, Arunava Biswas2, Dipasri Bhattacharjee3
1 Associate Professor, Department of Anaesthesiology, Murshidabad Medical College and Hospital, Berhampur, West Bengal, India.
2 Associate Professor, Department of Pharmacology, Coochbehar Government Medical College and Hospital, Coochbehar, West Bengal, India.
3 Professor, Department of Anaesthesiology, R.G. Kar Medical College and Hospital, Kolkata, West Bengal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Arunava Biswas, Department of Pharmacology, Coochbehar Government Medical College, Coochbehar-736101, West Bengal, India.
E-mail: drabiswas@gmail.com
Introduction
Spinal anaesthesia is an accepted technique in obstetric surgery but often associated with hypotension and nasal congestion. Phenylephrine, α1 agonist has been tried earlier in managing such adverse situations by parenteral route.
Aim
To compare the effect of prophylactic phenylephrine nasal drop versus normal saline as nasal drop in the prevention of hypotension and nasal congestion during spinal anaesthesia in emergency Lower Uterine Caesarean Section (LUCS).
Materials and Methods
A prospective randomised double blinded study was conducted on 90 parturient over a period of five months. Patients were randomised into Group A (n=46) phenylephrine (3 μg/kg) nasal drop (10%) and Group B (n=44) received normal saline nasal drop apart from all other standard pre-anaesthetic medications. Blood pressure was measured and nasal congestion recorded every two minutes in the first five minutes and then every five minutes until 30 minutes after spinal anaesthesia. Numerical variables were analysed using independent student’s t-test.
Results
The incidence of hypotension was significantly prevented for those participants with prophylactic phenylephrine (p<0.001) compared to the normal saline group. Systolic and diastolic blood pressures parameters after administration of spinal anaesthesia were maintained well in the phenylephrine group as compared to the normal saline group at various time intervals. Moreover, there were no incidences of nasal congestion among the parturient in the phenylephrine arm.
Conclusion
Prophylactic phenylephrine nasal drop remarkably reduced the incidence of spinal anaesthesia induced hypotension and nasal congestion compared to normal saline treated group. Such approach can be recommended in emergency LUCS provided more robust data are generated from large multicentric studies.
Emergency caesarean section, Intrathecal anaesthesia, Low blood pressure, Nasal stuffing, Phenylephrine prophylaxis
Introduction
Spinal anaesthesia is very common approach by the anaesthetist for obstetric patient in emergency LUCS to avoid the risks of general anaesthesia related to difficult intubation and aspiration of gastric contents [1-3]. Post spinal hypotension is a common side effect of subarachnoid block [4,5]. It occurs due to the drop of systemic blood pressure as a result of systemic vasodilatation secondary to sympathetic blockade by intrathecal administration of local anaesthetics. There is a reduction in systemic vascular resistance due to sympathetic blockade accompanied by the high level of sympathetic block. Also, physiologically there is aorto-caval compression by gravid uterus, as well as decreased systemic vascular resistance which altogether cause hypotension [6,7].
For the prevention, treatment or lessening of hypotension like adverse effect of post spinal anaesthesia varying pharmacological and nonpharmacological methods are available [8-11]. However, nonpharmacologic mechanisms are associated with high incidence of high spinal block and unilateral block especially when hyperbaric local anaesthetics are used [12].
For determining the effectiveness of drugs like ephedrine, phenylephrine, adrenaline, metaraminol etc., and to prevent and control hypotension many studies have been conducted so far [13,14]. However, any gold standard therapy for prevention and control of post spinal hypotension is still lacking.
Phenylephrine is a selective α1-adrenergic receptor agonist which has been used as an alternative vasopressor intravenously to increase blood pressure. The property of sympathomimetic effect in absence of β-adrenergic activity does not increase the force of contraction of cardiac muscles as well as cardiac output. On the other hand, there might be an increase in blood pressure leading to slow heart rate i.e., reflex bradycardia due to stimulation of vascular baroreceptor when administered intravenously [15,16].
The authors of this study didn’t find any publication on its use through intranasal route during spinal anaesthesia to prevent nasal congestion as well as to prevent hypotension. Topical administration of phenylephrine as nasal decongestants and mydriatic in approved indications are well established. It was also observed that even 4 to 30 times increase in such recommended dose of phenylephrine didn’t have significant effect of blood pressure and heart rate [17]. Even in 1976, oral phenylephrine was approved for non-prescription use as a decongestant by the Food and Drug Administration (FDA) [18].
Since intranasal administration of any drug for a therapeutic indication is much more hassle free and more patient friendly than parenteral administration and no previous study indicating any work in this field, this study was attempted primarily to determine the efficacy of prophylactic intranasal use of phenylephrine as compared to a placebo like normal saline in prevention of regional anaesthesia induced hypotension and secondarily, prevention of nasal congestion in emergency LUCS.
Materials and Methods
A randomised double blinded study was conducted on 96 parturient aged 20 to 35 years with American Society of Anaesthesiologists (ASA) class physical status- Grade I and II undergoing emergency LUCS under spinal anaesthesia at Murshidabad Medical College and Hospital, Berhampore, West Bengal, India for a period of five months (March-July 2019).
Parturient who refused to participate in the study, having difficulty in communication, history of study drug allergy or receiving any nasal decongestant within 24 hours prior to surgery or having prior nasal congestion were excluded from the study.
After prior approval {MMC-IEC/2019/15(3) dated 16.02.2019} from the Institutional Ethics Committee, the parturient those who were selected according to inclusion/exclusion criteria, randomised into two groups using online random number generator software Random®. Written informed consent were obtained from them and divided into Group A who received phenylephrine (3 μg/kg) PARIFER® nasal drop (10%) and Group B received normal saline NASOCLEAR® nasal drop, respectively after administration of routine pre-anaesthetic medications, preloading with 10 mL/kg of ringer lactate and spinal anaesthesia.
An independent anaesthetist induced the spinal anaesthesia (Intrathecal bupivacaine heavy 10-12.5 mg) as well as the study drugs and didn’t participate in the clinical assessment of the parturient or analysis of their data. Patients as well as the assessor were blinded regarding group allocation of the study patient.
Blood pressure and nasal congestion was measured and recorded every two minutes in the first five minutes and then every five minutes until 30 minutes after spinal anaesthesia and if a decrease in systolic blood pressure to 80% of baseline happened, the rate of infusion crystalloid was increased and vasopressor was used. Oxygen was used for patients as 4-6 L/min via a face mask. The average change in systolic and diastolic blood pressure, the frequency of nausea and vomiting and presence of nasal congestion were measured and recorded during the surgery.
Statistical Analysis
After completion of data collection, they were checked for error (if any). The data were entered to SPSS version 20.0 statistical package and analysis was performed. Numerical variables were analysed using independent student’s t-test. Incidence of hypotension was compared using chi-square test. The p-value <0.05 was considered statistically significant. Numerical data were presented as mean±Standard deviation or median (IQR) and categorical data as proportions (%). Baseline characteristics and risk levels were checked for similarity in the groups. The flow chart of the study is depicted in [Table/Fig-1].
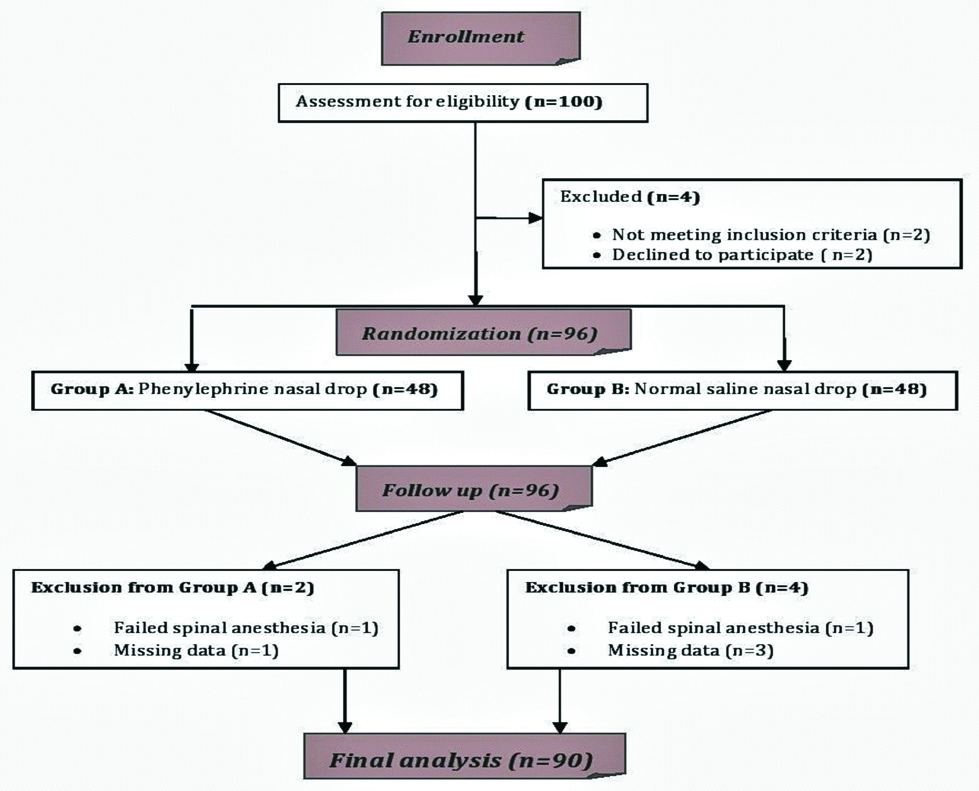
Results
After an initial screening of 100 parturient, a total (n=96) were given spinal anaesthesia. Two patients were excluded because spinal anaesthesia failed and converted to general anaesthesia and four patients were excluded due to missing data from both the group. Finally, n=90 labouring mothers who underwent emergency LUCS under spinal anaesthesia during the study period were included and analysed. The indications for caesarean section were cephalopelvic disproportion, non-reassuring foetal heart rate, failed induction, etc.
The mean age of patients was 21.73±2.44 years in Group A and 22.20±4.03 years in Group B. The mean Body Mass Index (BMI) was 26.73±2.88 kg/m2 in the group A and 27.96±1.60 kg/m2 in the group B. According to t-test, there was no statistically significant difference between the two groups in terms of age and BMI [Table/Fig-2].
Comparison mean of age and body mass index in the two groups.
| Demographic parameter | Group | Mean±SD | p-value |
|---|
| Age (years) | A (n=46) | 21.73±2.44 | 0.84 |
| B (n=44) | 22.20±4.03 |
| BMI (kg/m2) | A (n=46) | 26.73±2.88 | 0.65 |
| B (n=44) | 27.96±1.60 |
There was a significant difference when the mean arterial blood pressure was compared between the two study groups. There was a very significant fall (p=0.001) in systolic as well as diastolic blood pressure in the normal saline group at an interval of 3, 5, 10, 15, 20 minutes and significant change at 30 minute time interval when compared with phenylephrine group [Table/Fig-3].
Mean systolic and diastolic blood pressure measured between the two study groups.
| Time (min) | Systolic (mm Hg)# | Diastolic (mm Hg)# |
|---|
| Group A (n=46) | Group B (n=44) | p | Group A (n=46) | Group B (n=44) | p |
|---|
| Basal* | 124.14±13.76 | 126.77±7.21 | 0.54 | 74.15±15.46 | 75.62±11.98 | 0.67 |
| 1** | 120.76±7.89 | 110±10.63 | 0.05 | 70±11.50 | 72±8.90 | 0.45 |
| 3** | 118.45±8.45 | 102±8.90 | 0.001 | 68±8.97 | 58±7.20 | 0.01 |
| 5** | 110.66±1.82 | 92±11.67 | 0.001 | 70±10.37 | 56±9.37 | 0.001 |
| 10** | 106.12±11.56 | 88±15.83 | 0.001 | 72±9.80 | 55±6.23 | 0.001 |
| 15 | 112±16.89 | 90±9.06 | 0.001 | 78±7.82 | 56±9.65 | 0.001 |
| 20 | 118±9.77 | 96±8.61 | 0.001 | 76±6.98 | 60±8.33 | 0.001 |
| 30 | 120±15.28 | 104±12.58 | 0.05 | 76±10.78 | 64±8.20 | 0.05 |
*Before administration of study drugs; **Time interval following administration of the study drugs; #Mean±SD
When the study parturient was asked about their nasal congestion status only 4.44% (n=2) patient in the phenylephrine group as compared to 100% in the normal saline group complained. Although 8.88% (n=4) parturient in the group A complained of some sort of nasal irritation which was absent in the normal saline group [Table/Fig-4].
Comparison of nasal congestion among the parturient in the study groups.
| Nasal congestion | Group A (n=46) | Group B (n=44) |
|---|
| Yes | 2 | 44 |
| No | 44 | 0 |
Discussion
Phenylephrine is a directly acting sympathomimetic synthetic drug usually applied for induction of local vasoconstriction. Its use in decongestant nasal sprays [17] and mydriatics [19] does not bring any significant change in cardiovascular system at its therapeutic dose. Furthermore, regional phenylephrine injection often considered for management of localise increase in blood flow like patients of priapism [20]. Parenteral administration of phenylephrine acts as an effective vasopressor in major surgeries like caesarean section and cardiovascular surgeries [21].
Oral administration of phenylephrine has a marked first pass metabolism decreasing its duration of action on the other hand intravenous route has its own demerits although it by passes liver metabolism. Therefore, absence of data about intranasal route of administration of phenylephrine which according to our search never attempted earlier as prophylactic vasoconstrictive agent for prevention of hypotension along with its established therapeutic indication i.e., nasal decongestant provoked us to attempt this study.
It was observed that the incidence of hypotension was much lesser at 3, 5, 10, 15, 20 minutes after administration of the spinal anaesthesia in the prophylactic phenylephrine nasal drop Group A when compared with normal saline nasal drop Group B. Additionally, there was no incidence of nasal congestion in group A as compared to the other group which certainly benefitted the parturient. Husseini H et al., compared the effect of mucosal phenylephrine and IV ephedrine on the prevention of hypotension following spinal anaesthesia. In the study, they did not observe any difference in the incidence of hypotension between the two groups [22]. Magalhães E et al., evaluated the impact of ephedrine and phenylephrine on the prevention of hypotension in spinal anaesthesia for caesarean section as well as its effects on foetus and found that ephedrine is more effective in the prevention of hypotension than phenylephrine which was different from what was observed in present study [23]. Unlike these studies which were done on parenteral use of phenylephrine this study tried to find the effectiveness of more patient friendly approach i.e., intranasal administration of phenylephrine in parturient and the outcome appeared to be quite encouraging. Clearly intranasal phenylephrine reached the systemic circulation in sufficient amount as appeared from this study which significantly prevented the hypotensive crisis of spinal anaesthesia.
Limitation(s)
This study was done on a limited cohort of 90 parturient for emergency LUCS, at a single centre. Comparison of efficacy and safety between intravenous vis a vis intranasal administration of phenylephrine as prophylactic use in prevention of perioperative hypotension was not attempted in this study which opens the door for future research. The pharmacokinetic profile of the phenylephrine was also not estimated which could have precisely indicated the bioavailability of the drug during present study. To attain external validity, accurate and acceptable results, various surgical procedures are to be incorporated in the study to substantiate the current findings.
Conclusion(S)
Incidence of hypotension was high during spinal anaesthesia given for emergency LUCS which can be efficiently controlled by intranasal administration of phenylephrine at the recommended dose. Additionally, it also reduces the nasal congestion of the parturient often associated with such situation.
Contribution(s)
SR- Concept and design of the study, Conduct of the study and data collection. AB- Concept and design of the study, manuscript preparation, statistically analysis and interpretation. DB- Critical revision of the manuscript review of the study, data collection.
*Before administration of study drugs; **Time interval following administration of the study drugs; #Mean±SD
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jan 31, 2020
Manual Googling: Mar 17, 2020
iThenticate Software: Mar 29, 2020 (14%)
[1]. Habib AS, A review of the impact of phenylephrine administration onmaternal hemodynamics and maternal and neonatal outcomes in women undergoing caesarean delivery under spinal anaesthesiaAnaesth Analg 2012 114:377-90.10.1213/ANE.0b013e3182373a3e22104076 [Google Scholar] [CrossRef] [PubMed]
[2]. Mwaura LW, Mung’ayi V, Kabugi J, Mir S, A randomised control trial comparing weight adjusted dose versus fixed dose prophylactic phenylephrine infusion on maintaining systolic blood pressure during caeserean section under spinal anaesthesiaAfr Health Sci 2016 16:399-41.10.4314/ahs.v16i2.827605955 [Google Scholar] [CrossRef] [PubMed]
[3]. Brenck F, Hartmann B, Katzer C, Obaid R, Brüggmann D, Benson M, Hypotension after spinal anaesthesia for caesarean section: Identification of risk factors using an anaesthesia information management systemJ Clin Monit Comput 2009 23:85-92.10.1007/s10877-009-9168-x19277879 [Google Scholar] [CrossRef] [PubMed]
[4]. Allen T, George R, White W, Muir H, Habib A, A double-blind, placebo-controlled trial of 4 fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anaesthesia for caesarean deliveryObst Anaesth Dig 2011 31:20410.1097/01.aoa.0000406652.03821.09 [Google Scholar] [CrossRef]
[5]. Kee WDN, Khaw KS, Ng FF, Prevention of hypotension during spinal anaesthesia forcaesarean delivery- An effective technique using combination phenylephrine infusion and crystalloid co-hydrationAnaesthesiology 2005 103:744-50.10.1097/00000542-200510000-0001216192766 [Google Scholar] [CrossRef] [PubMed]
[6]. Stewart A, Fernando R, McDonald S, Hignett R, Jones T, The dose dependent effects of phenylephrine for elective caesarean deliveryunder spinal anaesthesiaAnaesth Analg 2010 111:1230-37.10.1213/ANE.0b013e3181f2eae120841418 [Google Scholar] [CrossRef] [PubMed]
[7]. Sakata K, Yoshimura N, Kito K, Tanabe K, Iida H, Prediction of hypotension during spinal anaesthesia for elective caesarean section by altered heart rate variability induced by postural changeInt J of Obstet Anaesth2017(29):34-38.10.1016/j.ijoa.2016.09.00427789074 [Google Scholar] [CrossRef] [PubMed]
[8]. Bagle AA, Vishnu A, Kumar A, Malik A, Garg V, Evaluation of leg wrapping forthe prevention of postspinal hypotension in caesarean section under spinal anaesthesiaAnaesth Essays Res 2017 11:439-43.10.4103/0259-1162.19456428663637 [Google Scholar] [CrossRef] [PubMed]
[9]. Heesen M, Klimek M, Hoeks SE, Rossaint R, Prevention of spinal anaesthesia-induced hypotension during caesarean delivery by 5-hydroxytryptamine-3 receptor antagonists: A systematic review and metaanalysis and metaregressionAnaesth Analg 2016 123:977-88.0.1213/ANE.000000000000151127537930 [Google Scholar] [CrossRef] [PubMed]
[10]. Sng BL, Tan HS, Sia AT, Closed-loop double-vasopressor automated system vsmanual bolus vasopressor to treat hypotension during spinal anaesthesia for Caesarean section: A randomised controlled trialAnaesthesia 2014 69:37-45.10.1111/anae.1246024256483 [Google Scholar] [CrossRef] [PubMed]
[11]. Melchor JR, Espinosa Á, Hurtado EM, Francés RC, Pérez RN, Gurumeta AA, Colloids versus crystalloids in the prevention of hypotension induced by Spinal anaesthesia in elective caesarean sectionA systematic review and meta-analysisMinerva Anestesiol 2015 81:1019-30. [Google Scholar]
[12]. Kinsella SM, Lateral tilt for pregnant women: Why 15 degrees?Anaesthesia 2003 58:835-36.10.1046/j.1365-2044.2003.03397.x12911353 [Google Scholar] [CrossRef] [PubMed]
[13]. Kee WDN, Khaw KS, Ng FF, Lee BB, Prophylactic phenylephrine infusion for preventing hypotension during spinal anaesthesia for caesarean deliveryAnaesth Analg 2004 98:815-21.0.1213/01.ANE.0000099782.78002.3014980943 [Google Scholar] [CrossRef] [PubMed]
[14]. Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G, Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in Caesarean sectionBr J Anaesth 2006 96:95-99.10.1093/bja/aei26516311286 [Google Scholar] [CrossRef] [PubMed]
[15]. Poterman M, Vos JJ, Vereecke HE, Struys MM, Vanoverschelde H, Scheeren TW, Differential effects of phenylephrine and norepinephrine on peripheral tissue oxygenation during general anaesthesia: A randomised controlled trialEur J Anaesthesiol 2015 32:571-80.10.1097/EJA.000000000000024725760679 [Google Scholar] [CrossRef] [PubMed]
[16]. Kuhn JC, Hauge TH, Rosseland LA, Dahl V, Langesæter E, Hemodynamics of phenylephrine infusion versus lower extremity compression during spinal anaesthesia for caesarean delivery: A randomised, double-blind, placebo-controlled studyAnaesth Analg 2016 122:1120-29.10.1213/ANE.000000000000117426991619 [Google Scholar] [CrossRef] [PubMed]
[17]. Myers MG, Iazzetta JJ, Intranasally administered phenylephrine and blood pressureC and Med Assoc J 1982 1(27):365-68. [Google Scholar]
[18]. Department of Health, Education, and Welfare, Food and Drug Administration Establishment of a monograph for OTC cold, cough, allergy, bronchodilator, and anti-asthmatic productsFed Regist 1976 41:38399-400. [Google Scholar]
[19]. Tanner V, Casswell AG, A comparative study of the efficacy of 2.5% phenylephrine and 10% phenylephrine in pre-operative mydriasis for routine cataract surgeryEye 1996 10:95-98.10.1038/eye.1996.158763311 [Google Scholar] [CrossRef] [PubMed]
[20]. Azocar Hidalgo G, Van Cauwelaert R, Castillo Cadiz O, Aguirre Aguirre C, Wohler Campos C, Treatment of priapism with phenylephrineArch Esp Urol 1994 47:785-87. [Google Scholar]
[21]. Ertmer C, Morelli A, Westphal M, (2009) The Role of Phenylephrine in Perioperative Medicine. In: Vincent JL. (eds)Yearbook of Intensive Care and Emergency Medicine. Yearbook of Intensive Care and Emergency Medicine. vol 2009 Berlin, HeidelbergSpringer10.1007/978-3-540-92276-6_46 [Google Scholar] [CrossRef]
[22]. Husseini H, Abbasi H, Heidari R, Comparing the effect of mucosal phenylephrine with intravenous ephedrine on the prevention and treatment of spinal anaesthesia-induced hypotensionJ Shahid Sadoughi Univ Med Sci Health Serv Yazd 2002 10(1):03-06. [Google Scholar]
[23]. Magalhães E, Govêia CS, de AraújoLadeira LC, Nascimento BG, Kluthcouski SM, Ephedrine versus phenylephrine: Prevention of hypotension during spinal block for caesarean section and effects on the fetusRev Bras Anestesiol 2009 59:11-20.10.1590/S0034-7094200900010000319374211 [Google Scholar] [CrossRef] [PubMed]