Introduction
The estimated prevalence of Hypomagnesemia in Type 2 Diabetes Mellitus (T2DM) ranges between 14% to 48% in various studies. Hypomagnesemia has been found to be associated with increased insulin resistance in patients of T2DM.
Aim
The aim was to estimate fasting serum magnesium levels in the patients of T2DM and to correlate serum magnesium with glycaemic status and nephropathy status.
Materials and Methods
It was a cross-sectional study conducted at KIMS, Bhubaneswar between October 2017 to September 2019 which included a total of 187 T2DM patients. The glycaemic status was assessed by HbA1c and subdivided into good (HbA1c <7%), intermediate (HbA1c 7-9%) and poor control (HbA1c >9%). The nephropathy status was assessed by urine Albumin-Creatinine Ratio (ACR) and subdivided into macroalbuminuria (Urine ACR >300 mg/g), microalbuminuria (Urine ACR 30-299 mg/g) and non-nephropathy (Urine ACR <30 mg/g) groups. Student t-test and One-way ANOVA test were used to find the significance of mean pattern of serum magnesium in different groups and Pearson correlation coefficient test was used to correlate serum magnesium with glycaemic and nephropathy status.
Results
Out of 187 patients, 49 (26.2%) patients were found to have hypomagnesemia. In the poor glycaemic status group, 30% of patients had hypomagnesemia as compared to 26% in the intermediate and 19% in the good glycaemic status group. In the macroalbuminuria group, 41.3% patients had hypomagnesemia as compared to 17.6% in the microalbuminuria group and 4.5% in the non-nephropathy group.
Conclusion
There was a weak negative correlation (r=-0.04) between serum magnesium and glycaemic status which was not statistically significant (p=0.62). There was a negative correlation (r=-0.17) between serum magnesium and nephropathy status which was found to be statistically significant (p=0.02).
Diabetic nephropathy, Hypomagnesemia, India, Insulin resistance, Microalbuminuria
Introduction
The number of patients with Diabetes Mellitus (DM) has been increased from 108 million in 1980 to 422 million in 2014. The global prevalence of DM in adults has risen from 4.7% in 1980 to 8.5% in 2014 [1]. Low magnesium levels have been reported to accelerate deterioration of kidney function [2].
Serum magnesium level in the normal individual is maintained between 0.7 to 1.0 mmol/L i.e., 1.68 to 2.4 mg/dL [3]. Low serum magnesium levels have been found to be associated with increased insulin resistance in patients of T2DM and metabolic syndrome [4]. Hypomagnesemia causes defective insulin receptor phosphorylation and this is considered the main mechanism leading to insulin resistance.
Insulin dependent Glucose Transporters (GLUT-4) is responsible for glucose uptake into the skeletal muscles and is responsible for metabolism of about 80% of dietary glucose load. Obese patients are prone to develop T2DM. Adipocytes release IL-1 (Interleukin-1) and Tumour Necrosis Factor-α (TNF-α) which are mediators of inflammation. This pro-inflammatory state results in insulin resistance and is responsible for high prevalence of diabetes mellitus in obese patients. Magnesium is an anti-inflammatory molecule and high levels of IL-1 and TNF-α are found in obese patients with hypomagnesemia [5]. Low magnesium also results in neutrophil activation and oxidative stress further aggravating insulin resistance [6]. The activity of glucokinase is regulated by Magnesium-ATP complex and thus, hypomagnesemia reduces the activity of glucokinase. Other enzymes of glycolysis and kreb’s cycle also depend on magnesium as a co-factor and thus, hypomagnesemia ultimately results in reduced glycolysis.
Deoxyribonucleic acid (DNA) synthesis and thereby, protein synthesis is dependent on intracellular magnesium concentration which is tightly regulated. The uptake is mediated by magnesium channels and transporters, especially Solute Carrier Family 41 member 1 (SLC41A1), Magnesium Transporter 1 (MagT1) and Transient Receptor Potential Melastatin type 6 and 7 (TRPM6 and TRPM7). Single Nucleotide Polymorphisms (SNPs) in TRPM6 and SLC41A1 have been associated with increased risk of Type 2 DM [7,8]. TRPM6 channels are responsible for re-absorption of magnesium from distal tubules. TRPM6 receptors are tightly regulated by magnesium levels, ATP availability and Epidermal Growth Factor (EGF). The mechanism of magnesium excretion from the Distal Convoluted Tubule (DCT) is still debated but recent studies have suggested that this mechanism to be facilitated by SLC41A1-sodium-magnesium exchanger [9].
Hyperglycaemia in diabetic patients results in hyper-filtration and increased renal urinary flow [10]. Increased urine volume in diabetic patients results into dilution of magnesium concentration in the pro-urine thereby disturbing the trans-epithelial magnesium gradient between the tubule and the interstitium resulting in increased magnesium excretion. Thus, magnesium reabsorption in Thick Ascending Limb (TAL) and DCT is inversely correlated with the urine volume in diabetic patients.
One of the major contributors in the mechanism of diabetic nephropathy is oxidative stress. Oxidative stress reduces TRPM6 activity and thereby, reduces magnesium reabsorption. This also contributes to hypomagnesemia in diabetic patients [11]. Methionine Sulfoxide Reductase B1 (MSRB1) has been found to recover the TRPM6 activity thereby, preventing oxidative stress induced damage to the kidneys.
Despite this humongous pool of diabetic patients, magnesium levels are not routinely checked in T2DM patients. Hypomagnesemia is associated with muscle cramps, hypokalemia, hypertension and these findings are often seen in T2DM patients. Widely used drugs such as thiazides, proton pump inhibitors and calcineurin inhibitors are responsible for risk of hypomagnesemia. Hence, patients taking these drugs should be closely monitored [12,13].
Some studies had varying results in the past, ranging from 10-48% prevalence of hypomagnesemia in T2DM patients [14-25]. There have also been a few studies which have found a statistically significant negative correlation between serum magnesium and HbA1c [18,19,23,24]. To our knowledge, there have been no such studies in south-east India which led us to explore the prevalence of hypomagnesemia in T2DM patients in the present clinical setting and correlation of serum magnesium with glycaemic and nephropathy status. The aim of this study was to estimate fasting serum magnesium concentration in patients of T2DM and to find the correlation of serum magnesium with glycaemic and nephropathy status in these patients.
Materials and Methods
Study Design
It was a cross-sectional observational study conducted for a period of 24 months from October 2017 to September 2019. According to the hospital data, 5126 patients were admitted in the Department of Internal Medicine in the year 2016, out of which 728 patients had T2DM. Thus, the expected frequency (P) of T2DM was 728/5126=14.2%.
Using the standard formula for sample size calculation, with a margin of error (α) of 0.05, confidence interval (1-α) of 0.95, Z0.95 of 1.96 (normal distribution table) and absolute precision (D) of 5%, the sample size (n) was calculated to be 187 patients.
Inclusion Criteria: The patients for the study were selected from those admitted in the Department of Internal Medicine of Kalinga Institute of Medical Sciences, Bhubaneswar, India. The patients included in the study were ≥18 years of age and known cases of T2DM for more than 6 months.
Exclusion Criteria: Patients who had acute coronary syndrome in the last 3 months, non-diabetic Chronic Kidney Disease (CKD), chronic liver disease, chronic diarrhoea, epilepsy, known malignancies, patients on oral magnesium supplementation, antacids or proton pump inhibitors and pregnant or lactating mothers were excluded from the study.
Data was collected using a proforma meeting the objectives of the study. The cases for the study were selected in accordance with the above mentioned inclusion and exclusion criteria. The purpose of the study was explained to the patients and informed consent was obtained. Ethical approval was obtained from the Institutional Ethical Committee vide reference number KIMS/KIIT/IEC/69/2017 dated 15.09.2017. The glycaemic status was assessed by HbA1c and subdivided into good (HbA1c <7%), intermediate (HbA1c 7-9%) and poor control (HbA1c >9%) [26]. The nephropathy status was assessed by urine ACR and subdivided into macroalbuminuria (Urine ACR ≥300 mg/g), microalbuminuria (Urine ACR 30-299 mg/g) and non-nephropathy (Urine ACR <30 mg/g) groups [27]. Fasting serum magnesium was estimated in all the patients. Patients with serum magnesium of less than 1.7 mg/dL were classified as hypomagnesemia and more than 2.3 mg/dL were classified as hypermagnesemia [28].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6. Student t-test and One-way ANOVA test were used to find the significance of mean pattern of serum magnesium in different groups according to glycaemic and nephropathy status. Pearson correlation coefficient test was used to correlate between serum magnesium and glycaemic status. It was also used to correlate between serum magnesium and nephropathy status.
Results
A total of 187 T2DM patients were enrolled in the study. The mean age of the study population was 58.8±11.6 years. The eldest patient was 87 years and the youngest patient was 32-year-old. Seventy-one (37.9%) patients were females and 116 (62.1%) were males. Eighty-eight (47%) patients were from urban areas and 99 (53%) from rural areas. One hundred seventeen (62.6%) patients were between the age group of 51-70 years [Table/Fig-1].
Distribution of patients according to age (n=187).
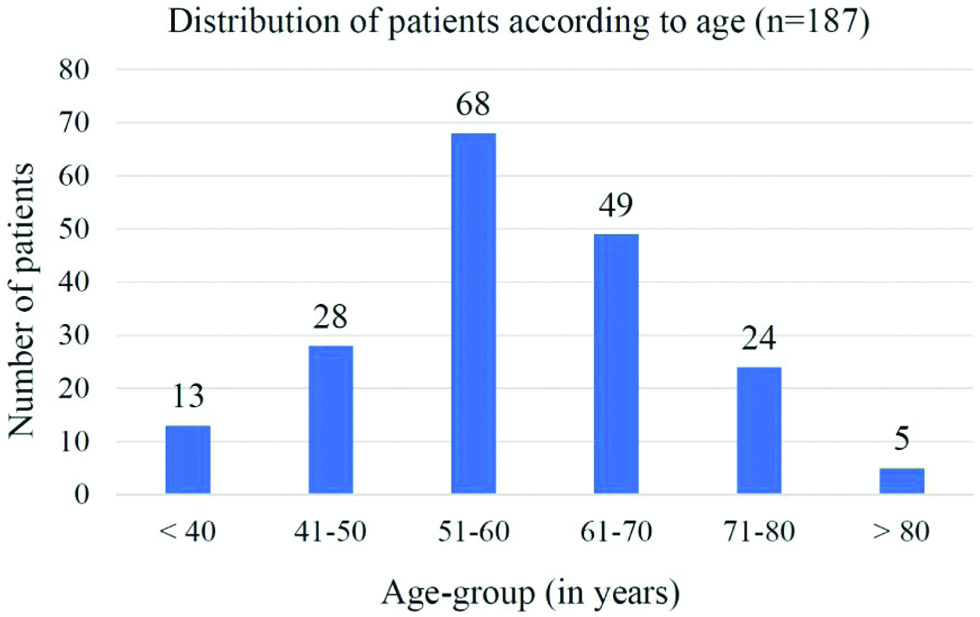
One hundred eighty-seven patients were divided on the basis of their glycaemic status as assessed by HbA1c [Table/Fig-2]. The mean age of patients was 62±11.3, 61.8±11.1 and 55.1±11 years in the good, intermediate and poor glycaemic status groups, respectively which was statistically significant (p=0.0003) but there was no statistically significant relation between the mean duration of T2DM in the 3 subgroups (p=0.71).
Mean FBS and PPBS according to HbA1c.
| HbA1c | Glycaemic status | Number of patients | Mean Fasting Blood Sugar (FBS) (mg/dL) | Mean Post Prandial Blood Sugar (PPBS) (mg/dL) |
|---|
| <7% | Good | 47 (25.1%) | 147.2±90.7 | 188.2±96.6 |
| 7-9% | Intermediate | 53 (28.3%) | 170.4±90.1 | 223.5±95.8 |
| >9% | Poor | 87 (46.6%) | 239.3±98.9 | 306.8±90.7 |
Fasting serum magnesium levels were estimated in 187 patients and the mean value was 1.89±0.34 mg/dL in the study population.
Out of 187 patients, 49 (26.2%) patients had hypomagnesemia [Table/Fig-3]. In the poor glycaemic status group, 30% of patients had hypomagnesemia as compared to 26% in the intermediate and 19% in the good glycaemic status group. None of the patients had hypermagnesemia.
Mean fasting serum magnesium according to HbA1c.
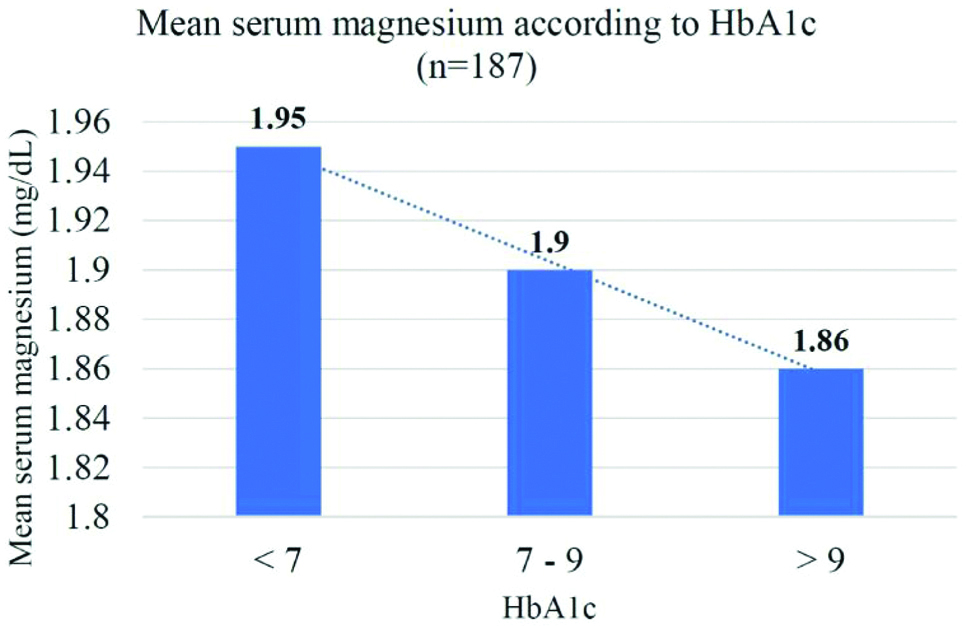
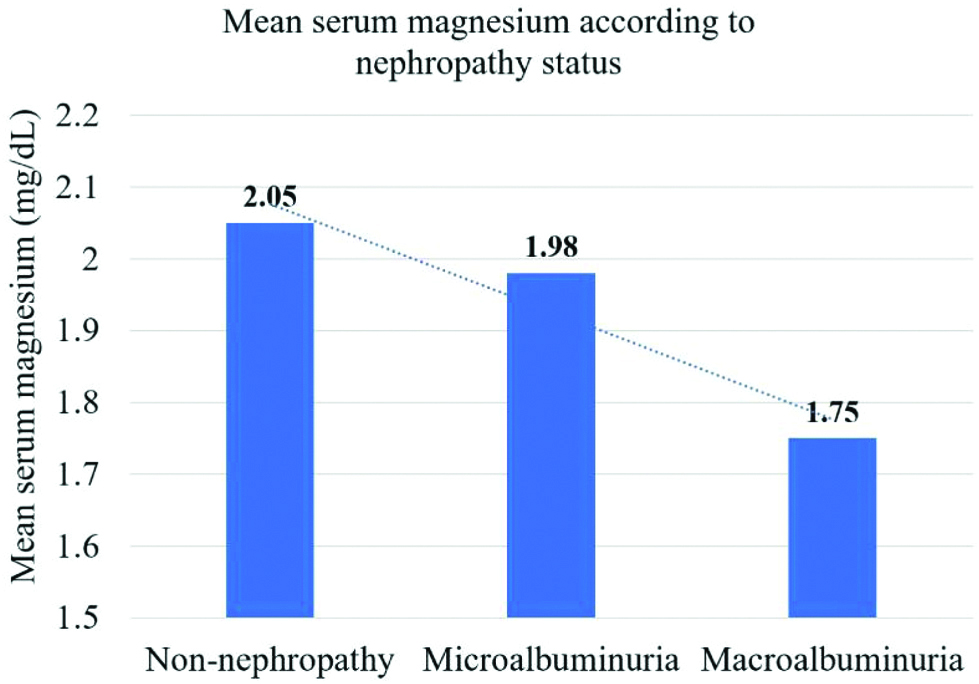
All the patients were also divided on the basis of their nephropathy status as assessed by Urine ACR [Table/Fig-4] and their serum magnesium level was estimated [Table/Fig-5]. In the macroalbuminuria group, 41.3% patients had hypomagnesemia as compared to 17.6% in the microalbuminuria group and 4.5% in the non-nephropathy group. There was no statistically significant relation between the mean age of patients in the 3 subgroups (p=0.32). The mean duration of T2DM was 5.9±6.1, 6.3±4.9 and 9.1±5.3 years in the non-nephropathy, microalbuminuria and macroalbuminuria groups, respectively which was statistically significant (p=0.0008).
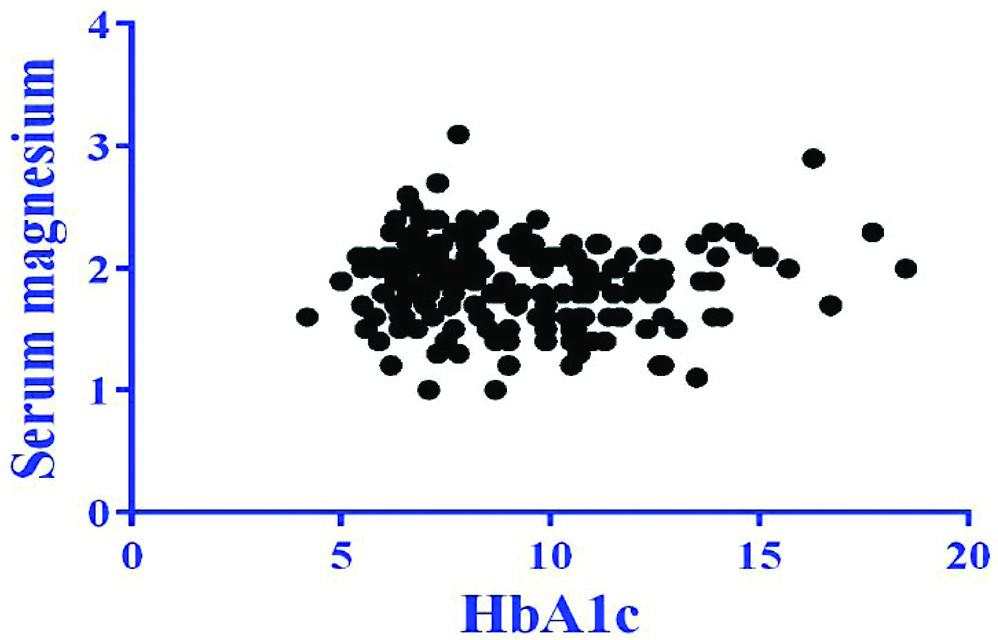
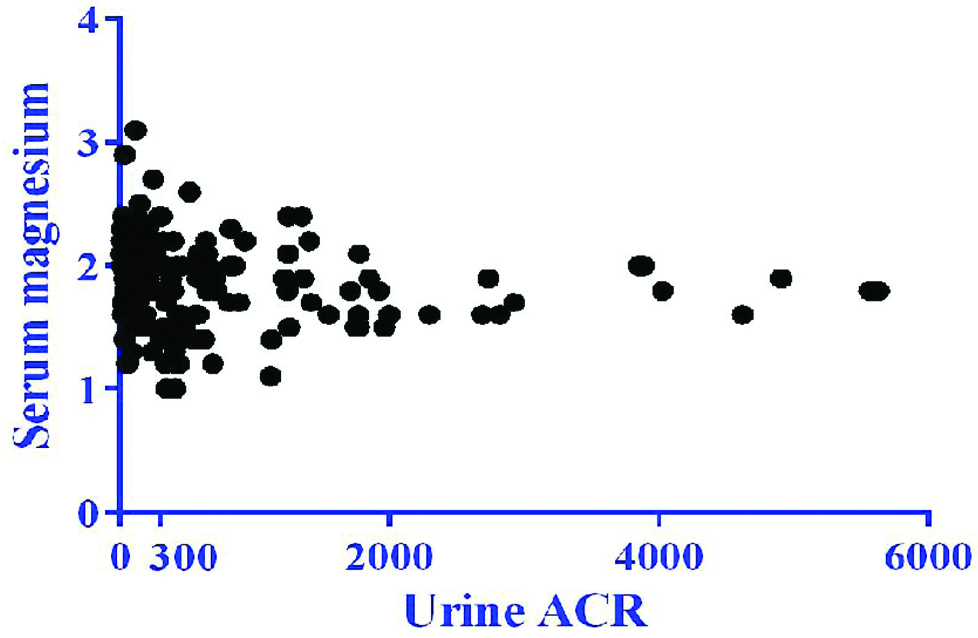
There was a weak negative correlation between serum magnesium and HbA1c which was not statistically significant (r=-0.04, p=0.62) [Table/Fig-6]. There was also a negative correlation between serum magnesium and urine ACR which was statistically significant (r=-0.17, p=0.02) [Table/Fig-7].
Mean FBS, PPBS and HbA1c according to Urine ACR.
| Urine ACR (in mg/g) | Nephropathy status | Number of patients | Mean Fasting Blood Sugar (FBS) (mg/dL) | Mean post Prandial Blood Sugar (PPBS)(mg/dL) | Mean HbA1c |
|---|
| <30 | Non-nephropathy | 22 (11.8%) | 174.5±87.9 | 220.8±98.6 | 7.9% |
| 30-299 | Microalbuminuria | 85 (45.4%) | 192.9±90.7 | 245.5±92.9 | 9.4% |
| ≥300 | Macroalbuminuria | 80 (42.8%) | 206.7±89.9 | 270.8±96.0 | 9.5% |
Mean fasting serum magnesium according to nephropathy status.
Correlation between serum magnesium and HbA1c.
r=-0.04; p=0.62
Correlation between serum magnesium and urine ACR.
r=-0.17; p=0.02
Discussion
In the present study, out of total 187 T2DM patients, 49 (26.2%) patients had hypomagnesemia. None of the patients had hypermagnesemia. The prevalence of hypomagnesemia was 19%, 26% and 30% in the good, intermediate and poor glycaemic status groups respectively. Subgroups with higher HbA1c levels reported a lower mean serum magnesium levels. This finding could be attributed to insulin resistance as insulin regulates magnesium reabsorption in the kidneys and further, low serum magnesium levels cause defect in insulin receptor phosphorylation thus causing a vicious cycle wherein there is worsening glycaemic status and hypomagnesemia.
Previous studies which have estimated hypomagnesemia in T2DM patients are summarised [Table/Fig-8] [14-23,25]. In a study conducted in Guwahati, India by Dasgupta A et al., there were 150 T2DM patients and the prevalence of hypomagnesemia was around 11% [14]. Similar study conducted in Turkey by Arpaci D et al., evaluated 673 diabetic patients and found 9.7% of them to have hypomagnesemia [18]. Another study by Parlapally RP et al., in Telangana, India estimated 38.6% patients to have hypomagnesemia in a cohort of 75 diabetic patients [20]. They also found that hypomagnesemia was associated with Diabetic Retinopathy and Nephropathy. In this study, hypomagnesemia was found in 26.2% of 187 patients. These wide differences in the prevalence of hypomagnesemia could be attributed due to the regional differences in lifestyle including exercise and diet which has significantly alter the glycaemic status and serum magnesium levels.
Previous studies on hypomagnesemia [14-23,25].
| Year | Place | Authors | Total number of patients | Percentage of hypomagnesemia |
|---|
| 2010 | Guwahati, India | Dasgupta A et al., [14] | 150 | 11% |
| 2012 | Osaka, Japan | Sakaguchi Y et al., [15] | 455 | 24% |
| 2012 | Barcelona, Spain | Lecube A et al., [16] | 200 | 48% |
| 2013 | Fremantle, Australia | Peters KE et al., [17] | 940 | 19% |
| 2015 | Sakarya, Turkey | Arpaci D et al., [18] | 673 | 9.7% |
| 2017 | Nijmegan, Netherlands | Kurstjens S et al., [19] | 395 | 31% |
| 2016 | Telangana, India | Parlapally RP et al., [20] | 75 | 38.6% |
| 2017 | Sagamu, Nigeria | Odusan OO et al., [21] | 125 | 23% |
| 2019 | Abbottabad, Pakistan | Noor MM et al., [22] | 180 | 34% |
| 2018 | Ankara, Turkey | Ilkay HO et al., [23] | 119 | 19% |
| 2019 | North India | Kumar P et al., [25] | 250 | 44% |
A study by Kumar P et al., (2019) observed that 110 (44%) out of 250 patients have hypomagnesemia [25]. They also found that magnesium deficiency was associated with poor glycaemic control and increased risk of diabetic retinopathy.
In this study, the prevalence of hypomagnesemia was 4.5%, 17.7% and 41.3% in the non-nephropathy, microalbuminuria and macroalbuminuria groups respectively. Subgroups with higher urine ACR reported lower mean serum magnesium levels which was statistically significant (p=0.001). These findings can be as a result of decline in magnesium reabsorption from the kidneys as the nephropathy progresses. There was also a rising trend seen in HbA1c in the 3 subgroups as the kidney function worsened, which was statistically significant (p=0.04).
In this study, there was a weak negative correlation between serum magnesium and glycaemic status (HbA1c) which was not statistically significant (r=-0.04, p=0.62). Similar studies were conducted in Turkey [18,23], Netherlands [19] and India [24] which suggested statistically significant correlation between serum magnesium and HbA1c in diabetic patients between -0.11 to -0.731 [Table/Fig-9] [18,19,23,24]. In this study, there was also a negative correlation between serum magnesium and nephropathy status (urine ACR) which was statistically significant (r=-0.17, p=0.02).
Previous studies on correlation between HbA1c and serum magnesium [18,19,23,24].
| Year | Place | Authors | Total number of patients | Pearson correlation co-efficient (r) | p-value |
|---|
| 2015 | Sakarya, Turkey | Arpaci D et al., [18] | 673 | -0.11 | 0.004 |
| 2017 | Nijmegan, Netherlands | Kurstjens S et al., [19] | 395 | -0.123 | 0.001 |
| 2019 | Ankara, Turkey | Ilkay HO et al., [23] | 119 | -0.309 | 0.001 |
| 2018 | Asaripallam, India | Marshnil RW and Mythili C [24] | 130 | -0.731 | 0.00 |
Limitation(s)
This study was hospital-based and not community-based. The serum magnesium, HbA1c and urine ACR were estimated in hospitalised patients of Type 2 Diabetes Mellitus. This study population might not be a complete representation of the patients living with Type 2 Diabetes Mellitus in the community.
Conclusion(s)
There was a weak negative correlation between serum magnesium and glycaemic status (HbA1c) which was not statistically significant. There was a negative correlation between serum magnesium and nephropathy status (urine ACR) which was found to be statistically significant. Further studies on role of oral supplementation of magnesium in improving the glycaemic status would help us to explore clinical utility of magnesium in counteracting insulin resistance in diabetic patients.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jan 11, 2020
Manual Googling: Mar 18, 2020
iThenticate Software: Mar 30, 2020 (11%)
[1]. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studiesLancet 2010 375(9733):2215-22.10.1016/S0140-6736(10)60484-9 [Google Scholar] [CrossRef]
[2]. Pham PCT, Pham PAT, Pham SV, Pham PT, Pham PMT, Pham PTT, Hypomagnesemia: A clinical perspectiveInt J Nephrol Renovasc Dis 2014 7:219-30.10.2147/IJNRD.S4205424966690 [Google Scholar] [CrossRef] [PubMed]
[3]. de Baaij JH, Hoenderop JG, Bindels RJ, Magnesium in man: Implications for health and diseasePhysiol Rev 2015 95(1):01-46.10.1152/physrev.00012.2014 [Google Scholar] [CrossRef]
[4]. Lima MdL, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, Serum and intracellular magnesium deficiency in patients with metabolic syndrome-Evidences for its relation to insulin resistanceDiabetes Res Clin Pract 2009 83(2):257-62.10.1016/j.diabres.2008.11.01919124169 [Google Scholar] [CrossRef] [PubMed]
[5]. Rodríguez-Morán M, Simental-Mendía LE, Gamboa-Gómez CI, Guerrero-Romero F, Oral magnesium supplementation and metabolic syndrome: A randomized double-blind placebo-controlled clinical trialAdv Chronic Kidney Dis 2018 25(3):261-66.10.1053/j.ackd.2018.02.01129793665 [Google Scholar] [CrossRef] [PubMed]
[6]. Bussière FI, Gueux E, Rock E, Girardeau JP, Tridon A, Mazur A, Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentrationBr J Nutr 2002 87(2):107-13.10.1079/BJN200149811895162 [Google Scholar] [CrossRef] [PubMed]
[7]. Chan KHK, Chacko SA, Song Y, Cho M, Eaton CB, Wu W-CH, Genetic variations in magnesium-related ion channels may affect diabetes risk among African American and Hispanic American womenJ Nutr 2015 145(3):418-24.10.3945/jn.114.20348925733456 [Google Scholar] [CrossRef] [PubMed]
[8]. Song Y, Hsu YH, Niu T, Manson JE, Buring JE, Liu S, Common genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (TRPM6 and TRPM7), magnesium intake, and risk of type 2 diabetes in womenBMC Med Genet 2009 10:410.1186/1471-2350-10-419149903 [Google Scholar] [CrossRef] [PubMed]
[9]. Kolisek M, Nestler A, Vormann J, Schweigel-Röntgen M, Human gene SLC41A1 encodes for the Na+/Mg2+ exchangerAm J Physiol Cell Physiol 2012 302(1):C318-26.10.1152/ajpcell.00289.201122031603 [Google Scholar] [CrossRef] [PubMed]
[10]. Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ, The impact of hyperfiltration on the diabetic kidneyDiabetes Metab 2015 41(1):05-17.10.1016/j.diabet.2014.10.00325457474 [Google Scholar] [CrossRef] [PubMed]
[11]. Cao G, Lee KP, Van Der Wijst J, De Graaf M, Van Der Kemp AM, Bindels RJM, Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stressJ Biol Chem 2010 285(34):26081-87.10.1074/jbc.M110.10365520584906 [Google Scholar] [CrossRef] [PubMed]
[12]. Hess MW, Hoenderop JGJ, Bindels RJM, Drenth JPH, Systematic review: Hypomagnesaemia induced by proton pump inhibitionAliment Pharmacol Ther 2012 36(5):405-13.10.1111/j.1365-2036.2012.05201.x22762246 [Google Scholar] [CrossRef] [PubMed]
[13]. Lameris AL, Monnens LA, Bindels RJ, Hoenderop JGJ, Drug induced alterations in Mg2+ homoeostasisClin Sci 2012 123(1):01-14.10.1042/CS2012004522409531 [Google Scholar] [CrossRef] [PubMed]
[14]. Dasgupta A, Saikia UK, Sarma D, Hypomagnesemia in type 2 diabetes mellitusIndian J Endocrinol Metab 2012 16(6):1000-03.10.4103/2230-8210.10302023226651 [Google Scholar] [CrossRef] [PubMed]
[15]. Sakaguchi Y, Shoji T, Hayashi T, Suzuki A, Shimizu M, Mitsumoto K, Hypomagnesemia in type 2 diabetic nephropathy: A novel predictor of end-stage renal diseaseDiabetes Care 2012 35(7):1591-97./10.2337/dc12-022622498805 [Google Scholar] [CrossRef] [PubMed]
[16]. Lecube A, Baena-Fustegueras JA, Fort JM, Pelegrí D, Hernández C, Simó R, Diabetes is the main factor accounting for hypomagnesemia in obese subjectsPLoS One 2012 7(1):e3059910.1371/journal.pone.003059922291997 [Google Scholar] [CrossRef] [PubMed]
[17]. Peters KE, Chubb SAP, Davis WA, Davis TME, The Relationship between hypomagnesemia, metformin therapy and cardiovascular disease complicating type 2 diabetes: The Fremantle Diabetes StudyPLoS One 2013 8(9):e7435510.1371/journal.pone.007435524019966 [Google Scholar] [CrossRef] [PubMed]
[18]. Arpaci D, Tocoglu AG, Ergenc H, Korkmaz S, Ucar A, Tamer A, Associations of serum magnesium levels with diabetes mellitus and diabetic complicationsHippokratia 2015 19(2):153-57.10.1530/endoabs.37.EP490 [Google Scholar] [CrossRef]
[19]. Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG, Determinants of hypomagnesemia in patients with type 2 diabetes mellitusEur J Endocrinol 2017 176(1):11-19.10.1530/EJE-16-051727707767 [Google Scholar] [CrossRef] [PubMed]
[20]. Parlapally RP, Kumari KR, Jyothi SA, Serum magnesium levels in Type 2 diabetes mellitusInt J Sci Stud 2016 4(5):176-79. [Google Scholar]
[21]. Odusan OO, Familoni OB, Odewabi AO, Idowu AO, Adekolade AS, Patterns and correlates of serum magnesium levels in subsets of Type 2 diabetes mellitus patients in NigeriaIndian J Endocrinol Metab 2017 21(3):439-42.10.4103/ijem.IJEM_190_1628553602 [Google Scholar] [CrossRef] [PubMed]
[22]. Noor MM, Nazir Q, Khan TM, Gillani S, Abbasi MA, Rauf A, Association between low serum magnesium level and type 2 diabetes mellitus in AbbottabadJ Ayub Med Coll Abbottabad 2019 31(2):226-29. [Google Scholar]
[23]. Ilkay HO, Sahin H, Tanriverdi F, Samur G, Association between magnesium status, dietary magnesium intake, and metabolic control in patients with type 2 diabetes mellitusJ Am Coll Nutr 2019 38(1):31-39.10.1080/07315724.2018.147619430160617 [Google Scholar] [CrossRef] [PubMed]
[24]. Marshnil RW, Mythili C, Study of serum magnesium levels and its correlation with glycated haemoglobin levels in type 2 diabetes mellitusIOSR Journal of Dental and Medical Sciences (IOSR-JDMS) 2018 17(6):22-27. [Google Scholar]
[25]. Kumar P, Bhargava S, Agarwal PK, Garg A, Khosla A, Association of serum magnesium with type 2 diabetes mellitus and diabetic retinopathyJ Family Med Prim Care 2019 8(5):1671-77.10.4103/jfmpc.jfmpc_83_1931198735 [Google Scholar] [CrossRef] [PubMed]
[26]. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK, Significance of HbA1c test in diagnosis and prognosis of diabetic patientsBiomark Insights 2016 11:95-104.10.4137/BMI.S3844027398023 [Google Scholar] [CrossRef] [PubMed]
[27]. Chida S, Fujita Y, Ogawa A, Hayashi A, Ichikawa R, Kamata Y, Levels of albuminuria and risk of developing macroalbuminuria in type 2 diabetes: Historical cohort studySci Rep 2016 6:2638010.1038/srep2638027210499 [Google Scholar] [CrossRef] [PubMed]
[28]. Cheungpasitporn W, Thongprayoon C, Qian Q, Dysmagnesemia in hospitalized patients: Prevalence and prognostic importanceMayo Clin Proc 2015 90(8):1001-10.10.1016/j.mayocp.2015.04.02326250725 [Google Scholar] [CrossRef] [PubMed]