Introduction
Oxidative stress has been associated with the pathogenesis of several diseases including cancer. Myrtenal, a monoterpene, has been shown to possess various therapeutic potentials including anti-inflammatory, hypolipidemic, antidiabetic and antioxidant properties.
Aim
The present study utilises the status of oxidative stress and detoxification cascade markers to explore the chemopreventive potential of myrtenal in experimental oral carcinogenesis induced in Golden Syrian hamsters.
Materials and Methods
Oral neoplasms were developed using 0.5% 7,12-dimethylbenz(a) anthracene (DMBA), three times a week for 14 weeks in the buccal pouch of Golden Syrian hamsters. the developed lesions were confirmed histopathologically as well differentiated squamous cell carcinoma. The status of oxidative stress markers {Thiobarbituric Acid Reactive Substances (TBARS)}, antioxidants {Superoxide Dismutase (SOD), Catalase (CAT), vitamin E, vitamin C, reduced glutathione (GSH), Glutathione Peroxidase (GPx)} and detoxification agents {cytochrome p450, cytochrome b5, Glutathione-S-Transferase (GST), Glutathione Reductase (GR), oxidised glutathione (GSSG), DT diaphorase} were determined using suitable colorimetric assays. The statistical comparison between the experimental groups was done by one-way analysis of variance followed by Duncan’s multiple range test.
Results
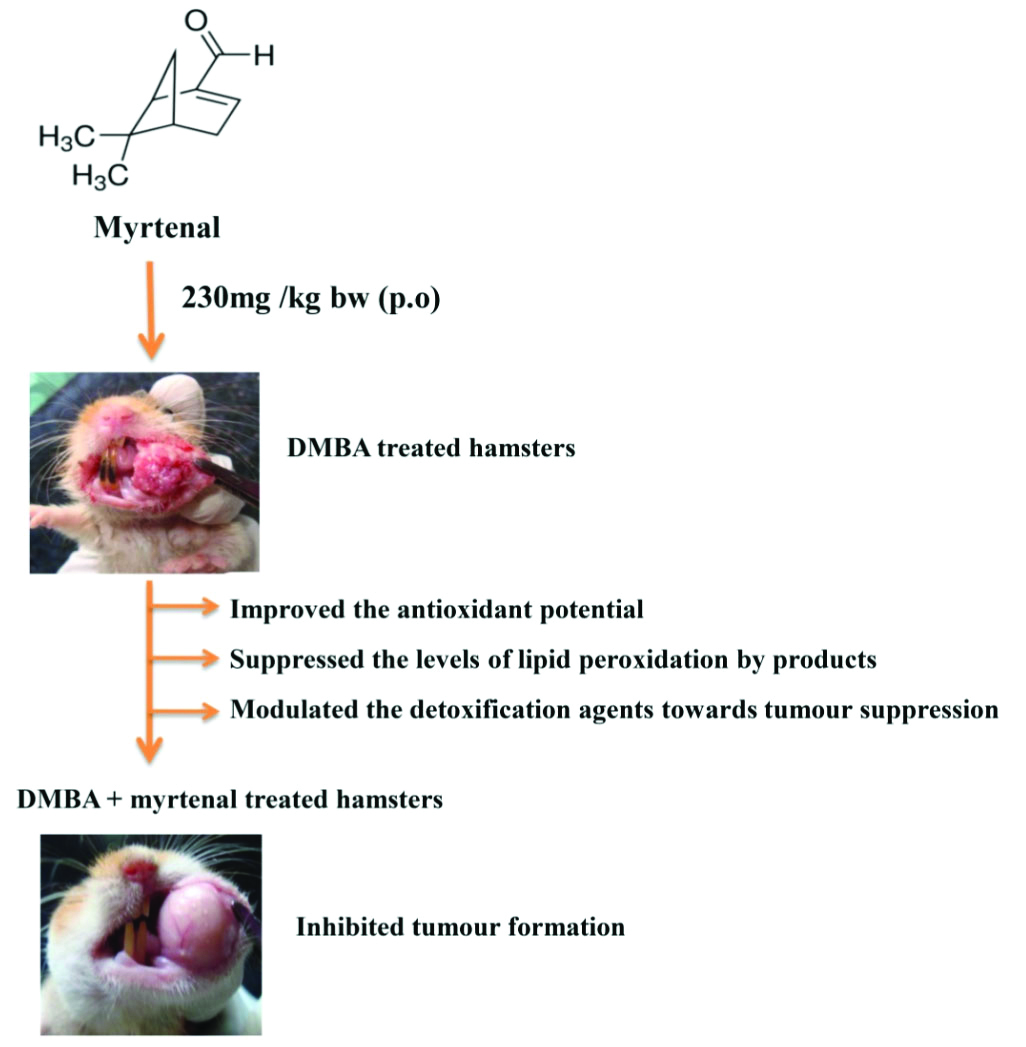
Topical application of DMBA not only resulted in tumour formation but also caused severe biochemical abnormalities in both plasma and buccal mucosa of Golden Syrian hamsters. Myrtenal administration (230 mg/kg bw p.o) to the hamsters painted with DMBA, suppressed or inhibited the formation of tumours and restored the status of oxidative stress markers and detoxification agents as well.
Conclusion
The present study thus concludes that the chemopreventive efficacy of myrtenal is either due to its anti-lipid peroxidative efficacy or due to its modulating effect on the detoxification agents towards the inhibition of oral carcinogenesis.
Introduction
Oral cavity cancer is one of the predominant cancers across the world and its prevalence and incidence is sharply increasing in several parts of the world, especially in Bangladesh, SriLanka and India [1]. Though multiple risk factors have been implicated in the oral cancer pathogenesis, the strongest risk factors associated with oral cancer development include consumption of tobacco products and alcohol abuse [2]. Histopathologically, 90% of the oral cancers are diagnosed as oral squamous cell carcinoma. Recent epidemiological and aetiological studies pointed out that unawareness on oral cancer risk factors and diagnostic delay are the two major factors, influencing the higher incidence of oral carcinoma worldwide [3].
In recent years, accumulating literatures focused the role of Reactive Oxygen Species (ROS) mediated oxidative DNA damage in the pathogenesis of several illness including diabetes, cardiovascular diseases and cancer [4-6]. Though ROS play a pivotal functions in various physiological and biochemical processes, their over production could lead to oxidative DNA adduct formation, which in turn leads to several pathological diseases [7]. Human body, however, has a vital antioxidant (enzymatic and non-enzymatic) defence mechanism to combat the adverse effects of ROS [8]. Imbalance in the oxidant and antioxidant status could thus lead to a pathological condition called oxidative stress, which is responsible for the pathogenesis of various illnesses. Human body has sophisticated detoxification mechanisms as well to detoxify the carcinogenic agents that enter the body. Liver phase I and phase II detoxification agents play a vital role in these processes and any defect in the status of phase I and phase II detoxification agents could thus lead to various pathological conditions including cancer [9-11].
The suppression, prevention or reversal of carcinogenesis using natural products or synthetic substances is termed as chemoprevention, which is now recognised worldwide as an appealing approach in experimental oncology [12]. Profound experimental studies explored the chemopreventive efficacies of a large number of medicinal plants, bioactive constituents and synthetic agents [13,14]. Previous experimental studies from our laboratory explored the anticancer effect of several medical plants and their bioactive principles and also analysed the status of oxidative stress markers during 7, 12-dimethylbenz (a) anthracene (DMBA) induced oral carcinogenesis [15,16].
DMBA has been recognised as an organ and site specific carcinoma and thus widely employed to induce several types of tumours in experimental animals, which include oral, skin and mammary cancers [17-19]. DMBA induces oral carcinogenesis via multiple mechanisms, which mainly involves the induction of oxidative stress, stimulation of chronic inflammatory response and by causing mutations in the DNA [20]. Due to biochemical and molecular similarities with human oral cancer, DMBA induced oral carcinoma was most commonly employed in experimental animals to assess the anticancer (chemopreventive) efficacy of natural products.
Monoterpenes, the bioactive constituent of several natural products, have been reported to possess diverse pharmacological properties. Myrtenal, a monoterpene, is the essential oil of pepper, mint and cumin. Profound studies have explored the therapeutic potential of myrtenal, which include antioxidant, anti-inflammatory and hypolipidemic effects [21]. Lokeshkumar B et al., reported that myrtenal suppressed experimental colon carcinogenesis through its antioxidant efficacy [22]. Myrtenal has been shown to possess membrane stabilising effects and played a vital role in the maintenance of cellular homeostasis [23]. Rathinam A and Pari L have shown the antidiabetic potential of myrtenal in experimental diabetes mellitus [24]. They suggested that myrtenal improved the secretion of insulin and activities of metabolising enzymes in streptozotocin induced diabetic rats. Hari Babu L et al., reported that the anticancer potential of myrtenal in diethylnitrosamine induced hepatocarcinogenesis [25]. They suggested that the anticancer effect of myrtenal might be due to its antioxidant and apoptotic induction potential. Babu LH et al., pointed out that the anticancer potential of myrtenal could be due to its ability to down regulate the expression of TNF-α in diethylnitrosamine induced hepato-carcinogenesis [26]. Though very few studies have demonstrated the anticancer potential of myrtenal in experimental cancer, there are no reports to scientifically validate the anticancer potential of myrtenal in DMBA induced hamster buccal pouch carcinogenesis. The present study, thus takes an intensive effort to explore the chemopreventive potential of myrtenal by assessing its antilipid peroxidative potential and modulating effect on detoxification agents in experimental oral carcinogenesis.
Materials and Methods
An experimental study was conducted on experimental animals, male Golden Syrian hamsters, which were obtained from National Institute of Nutrition, Hyderabad from January 2018 to May 2018. The experimental design was approved by Institutional Animals Ethical Committee as well (Proposal N0: 1175). The total duration of the experimental period was around 5 months from the acclimatisation period to the scarification of the animals (Acclimatisation period 14 days and experimental study duration 126 days). The sample size (30) taken was based on previous literatures [15,16]. The experimental animals were maintained in the Central Animal House, Annamalai University as per ethical principles and the animals were provided with food and water ad libitum. The age and weight of the animals were 7-8 weeks and 80-120 g, respectively. The experimental animals were divided into five groups and each group contained six animals. The experimental groups were categorised into five groups with more or less, similar mean weight. Group I served as vehicle treated control (liquid paraffin alone) and Groups II – V experimental animals were treated with DMBA, DMBA+myrtenal DMBA→myrtenal (denotes a chemotherapeutic phase. DMBA treatment first 10 weeks followed by 8 weeks myrtenal treatment) and myrtenal alone respectively. The experimental design is depicted in the [Table/Fig-1]. The animals were sacrificed by cervical dislocation after completing the experimental protocol [15,16].
Experimental design for chemoprevention study.

The biochemical studies were carried out in the plasma, liver and buccal mucosa of control and experimental animals. Histopathological studies were carried out in the buccal mucosa of control and experimental animals.
Tumour induction: Hamster’s buccal pouch exposed to DMBA painting 14 weeks (3 times a week) developed oral squamous cell carcinoma. Myrtenal at a dose of 230 mg/kg body weight was administrated orally to the experimental hamsters [25,26].
Biochemical estimations: The biochemical parameters that were analysed in the plasma (TBARS, SOD, CAT, vitamin E, vitamin C, GSH, GPx, liver (cytochrome p450, cytochrome b5, GSH, GST, GR, DT diaphorase) and buccal mucosa (TBARS, SOD, CAT, vitamin E, GSH, GPx, cytochrome p450, cytochrome b5, GST, oxidised glutathione (GSSG) are given in [Table/Fig-2] with the corresponding references [27-40].
The biochemical parameters that are analysed in the plasma, liver and buccal mucosa of experimental animals [27-40].
| Biochemical parameters | Samples | References |
|---|
| Thiobarbituric acid reactive substances (TBARS) | Plasma | Yagi K [27] |
| TBARS | Buccal mucosa | Ohkawa H et al., [28] |
| Superoxide dismutase (SOD) | Plasma and buccal mucosa | Kakkar PD and Viswanathan PN [29] |
| Catalase (CAT) | Plasma and buccal mucosa | Sinha AK [30] |
| Vitamin E | Plasma | Desai ID [31] |
| Vitamin E | Buccal mucosa | Palan PR et al., [32] |
| Vitamin C | Plasma | Omaye ST et al., [33] |
| Reduced glutathione (GSH) | Plasma, liver and buccal mucosa | Beutler E and Kelly BM [34] |
| Oxidised glutathione (GSSG) | Buccal mucosa | Tietze F [35] |
| Glutathione peroxidase (GPx) | Plasma and buccal mucosa | Rotruck JT et al., [36] |
| Cytochrome p450 | Liver and buccal mucosa | Omura T and Sato R [37] |
| Cytochrome b5 | Liver and buccal mucosa | Omura T and Sato R [37] |
| Glutathione-s-transferase (GST) | Liver and buccal mucosa | Habig WH et al., [38] |
| Glutathione reductase (GR) | Liver | Carlberg BM and Mannervik G [39] |
| DT-diaphorase | Liver | Ernster L [40] |
Statistical Analysis
The results obtained for the estimation of biochemical variables are expressed as mean±SD (n=6). The statistical significance between the experimental groups was analysed using ANOVA followed by DMRT. The p-value less than 0.05 between two different experimental groups were considered statistically significant. The SPSS software version 15.0 was used for the statistical analysis.
Results
The gross appearance of tumours and histopathological alterations were shown in [Table/Fig-3,4], respectively. DMBA induced tumour incidence and histopathological abnormalities in the buccal mucosa of experimental animals was shown in [Table/Fig-5,6], respectively. Topical application of DMBA alone (group II) on the buccal mucosa resulted in severe hyperplastic and dysplastic changes accompanied by well differentiated squamous cell carcinoma in the 14th week of experimental period. While myrtenal administration to hamsters painted with DMBA (group III) suppressed the tumour formation, mild hyperplastic and mild dysplastic changes were noticed in the buccal mucosa. Though 50% of hamsters in the chemotherapeutic phase (group IV; DMBA→myrtenal) developed oral tumours, the tumour size, volume and burden was very small as compared to DMBA alone treated hamsters (group II).
The gross appearance of buccal mucosa in control and experimental animals in each group.
a-Vehicle treated control: b-DMBA alone treated: c-DMBA+Myrtenal treated: d-DMBA→Myrtenal treated: e-Myrtenal alone treated

Histopathological features observed in the buccal mucosa of control and experimental animals in each group (H and E stain, 40x).
a. Control (normal epithelium):
b. DMBA alone keratin pearls
c. DMBA+Myrtenal (mild dysplastic epithelium)
d. DMBA→Myrtenal (severe dysplastic epithelium)
e. Myrtenal alone (normal epithelium)

Oral neoplasm incidence within expreimental hamsters (n=6).
| Parameter | Control | DMBA alone | DMBA+ Myrtenal | DMBA→ Myrtenal | Myrtenal alone |
|---|
| Oral tumour incidence | 0 | 100% | 0 | 50% | 0 |
| Total number of tumours | 0 | 19/6 | 0 | 4/3 | 0 |
| Tumour volume (mm3) | 0 | 216.87±16.60 | 0 | 32.41±2.47 | 0 |
| Tumour burden (mm3) | 0 | 686.755±52.31 | 0 | 43.213±3.29 | 0 |
Tumour volume was measured using the formula, v= (4/3)π (D1/2) (D2/2) (D3/2) where D1,D2 and D3 are the three diameter (mm3) of the tumour. Tumour burden was calculated by multiplying of tumour volume and number of tumours in animals.
Histopathological features observed in the buccal mucosa of control and experimental hamsters in each group (n=6).
| Parameter | Control | DMBA alone | DMBA+ Myrtenal | DMBA Myrtenal | Myrtenal alone |
|---|
| Hyperkeratosis | ns | Severe | Mild | Moderate | ns |
| Hyperplasia | ns | Severe | Mild | Moderate to severe | ns |
| Dysplasia | ns | Severe | Mild | Moderate to Severe | ns |
| Squamous cell carcinoma | ns | Large size, well differentiated squamous cell carcinoma observed in 100% of animals | Not observed | Small size, well differentiated squamous cell carcinoma observed in 50% of animals | ns |
ns: No significant change within epithelium
The biochemical alterations in the status of TBARS, antioxidants and phase I and phase II detoxification agents are depicted in the [Table/Fig-7,8] (plasma and buccal mucosa TBARS and antioxidants) and [Table/Fig-9,10] (liver and buccal mucosa detoxification agents). All the tabulated values are expressed as mean±Standard Deviation (SD) for six hamsters in each groups. Increase in TBARS levels with compromised antioxidants were noticed in the plasma of tumour bearing hamsters (group II). However, the present study observed a disturbances in the antioxidants status (vitamin E, GSH and GPx were increased; SOD and CAT were decreased) with the reduction in the TBARS levels in the buccal mucosa of tumour bearing hamsters. Similarly, a different pattern of phase I and phase II detoxification agents were noticed in the liver (phase I agents were increased; phase II agents were decreased) and buccal mucosa (phase I and II agents were increased) of tumour bearing hamsters. Oral administration of myrtenal to DMBA treated golden Syrian hamsters restored the status of TBARS, antioxidants and detoxification agents in the chemopreventive phase (group III) and improved the above said biomarkers status in the chemotherapeutic phase (group IV).
TBARS, SOD, CAT, vitamin E, vitamin C, GSH, GPx, in the plasma of control and experimental hamsters in each group (n=6).
Values that do not share a common superscript between two groups differ significantly at p<0.05 (DMRT). A-the amount of enzyme required to inhibit 50% NBT reduction; B-micromoles of hydrogen peroxide utilised/s; C-micromoles of glutathione utilised/min

TBARS, SOD, CAT, vitamin E, GSH, GPx in the buccal mucosa of control and experimental hamsters in each group (n=6).
Values that do not share a common superscript between two groups differ significantly at p<0.05 (DMRT). A-the amount of enzyme required to inhibit 50% NBT reduction; B-micromoles of hydrogen peroxide utilsed/s; C-micromoles of glutathione utilised/min

Phase I and phase II detoxification agents in the liver of control and experimental hamsters in each group (n=6).
Values that do not share a common superscript between two groups differ significantly at p<0.05 (DMRT). X-micromoles of cytochrome p450; Y-micromoles of cytochrome b5; Z-micromoles of 2,6-dichlorophenol reduced/min

Phase I and phase II detoxification agents in the buccal mucosa of control and experimental hamsters in each group (n=6).
Values that do not share a common superscript between two groups differ significantly at p<0.05 (DMRT). X-micromoles of cytochrome p450; Y-micromoles of cytochrome b5; Z-micromoles of 1-chloro 2,4 dinitrobenzene (CDNB)/reduced glutathione conjugate formed/min

Discussion
In the present study, tissue sections from liquid paraffin alone (Group I) treated hamsters showed uniform thickness stratified squamous epithelium. Single layer of thin keratin was seen. The basement membrane was well demarcated. The underlying connective fibrous tissues along with muscle fibres appeared normal [Table/Fig-4a]. Tissue sections from DMBA alone treated hamsters showed dysplastic epithelium with keratin pearls. The epithelium invaded the underlying connective tissues. The features like hyperkeratosis, hyperchromatism of the nucleus and increase in the mitotic figure were noticed [Table/Fig-4b]. Tissue sections from DMBA+Myrtenal treated hamsters (chemopreventive phase) showed hyperkeratotic uniform thickness stratified squamous epithelium. The basement membrane was well demarcated. The underlying connective fibrous tissues along with muscle fibres appeared normal [Table/Fig-4c]. Tissue sections from DMBA→Myrtenal treated hamsters (chemotherapeutic phase) showed dysplastic epithelium with basement membrane. Few areas were hyperkeratotic. Increase in the epithelial thickness was observed. The cells are hyperplastic with nuclear hyperchromatism. The underlying connective fibrous tissues with muscle fibers appeared normal [Table/Fig-4d]. Tissue sections from Myrtenal alone treated hamsters showed uniform thickness stratified squamous epithelium with thin layer of keratin. The cells appeared normal and the basement membrane was well demarcated. The underlying connective fibrous tissue with muscle fibres appeared normal [Table/Fig-4e].
Excessive formation of ROS and lack of antioxidants defence system were well documented in several types of cancers including oral cancer [41]. DMBA induced oxidative stress has been implicated as a major mechanism in the pathogenesis of oral carcinogenesis [42,43]. Both human and experimental oral cancer reported an altered status of lipid peroxidation and enzymatic and non-enzymatic defence mechanism in the plasma and buccal mucosa [42,44,45]. While enhanced plasma TBARS could be a reflection of diminished antioxidants, decreased buccal mucosa. TBARS could be due to higher utilisation of vitamin E and vitamin C by the tumour tissues.
Previous literatures have also documented higher production of superoxide and hydrogen peroxides in the tumour tissues, which could be the reason for the exhaustion of SOD and CAT in the tumour tissues [22,46]. Higher content of GSH accompanied by increased activity of GPx and lowered oxidised glutathione content reflected the role of GSH and GPx in the regulation of cellular proliferation. A few studies claimed high rate of cell proliferation and low PUFA content, a substrate of lipid peroxidation, could be the reason for the lowered TBARS levels in the tumour tissues [16,47].
Liver is the vital organ, which plays a pivotal role in the process of carcinogen detoxification via phase I and phase II detoxification cascade. Phase I detoxification agents such as cytochrome p450 and cytochrome b5 are involved in the metabolic activation of the xenobiotics [48]. The harmful products that are obtained during phase I reactions are excreted via phase II detoxification enzymes such as GST, GR and DT-diaphorase [49]. Lowered liver phase II detoxification agents in the tumour bearing animals clearly imply the accumulation of carcinogenic metabolites due to enhanced activities of phase I detoxification agents. Frequent topical application of DMBA to the buccal mucosa of DMBA alone treated hamsters could be the possible reason for the increases in the activities of both phase I and phase II detoxification agents.
Previous studies from our laboratory have explored the chemopreventive efficacy of several natural products in experimental oral carcinogenesis [15,16,42,50]. In the present study, myrtenal administration to DMBA treated hamsters restored the status of TBARS, antioxidants and detoxification agents as well as reverted the DMBA induced histopathological abnormalities, which clearly indicates the anticancer/chemopreventive potential of myrtenal during DMBA induced oral carcinogenesis. The anti lipid peroxidative efficacy and modulating effects on detoxification agents towards tumour inhibition could thus be the possible mechanism [Table/Fig-11] for the anticancer potential of myrtenal in DMBA induced oral carcinogenesis.
Possible mechanism of action of myrtenal.
Limitation(s)
In the present study, myrtenal exhibited a potent chemopreventive effect as evidenced by no tumour formation in hamsters treated with DMBA (Group III). However, the present study noticed tumour formation in 50% of the animals in the chemotherapeutic phase (Group IV). This might either be due to the dose fixed (230 mg/kg bw) for myrtenal or due to shorter treatment schedule (8 weeks) to the experimental animals. Further studies are warranted to evaluate the anticancer potential of myrtenal with a higher dose and should extend the treatment schedule for further 10 weeks to confirm the chemotherapeutic efficacy of myrtenal in experimental oral carcinogenesis.
Conclusion(s)
The chemopreventive potential of myrtenal has been explored in DMBA induced carcinogenesis. The said effects of myrtenal could be due to its anti-lipid peroxidative, antioxidant and modulating effects on detoxification agents. This study has examined the modulating effect of myrtenal on various molecular pathogenetic pathways related to oral carcinogenesis.
Tumour volume was measured using the formula, v= (4/3)π (D1/2) (D2/2) (D3/2) where D1,D2 and D3 are the three diameter (mm3) of the tumour. Tumour burden was calculated by multiplying of tumour volume and number of tumours in animals.
ns: No significant change within epithelium
[1]. Vigneswaran N, Williams MD, Epidemiologic trends in head and neck cancer and aids in diagnosisOral Maxillofac Surg Clin North Am 2014 26(2):123-41.10.1016/j.coms.2014.01.00124794262 [Google Scholar] [CrossRef] [PubMed]
[2]. Kumar M, Nanavati R, Modi TG, Dobariya C, Oral cancer: Etiology and risk factors: A reviewJ Can Res Ther 2016 12:458-63.10.4103/0973-1482.18669627461593 [Google Scholar] [CrossRef] [PubMed]
[3]. Abdulla R, Adyanthaya S, Kini P, Mohanty V, D’Souza N, Subbannayya Y, Clinicopathological analysis of oral squamous cell carcinoma among the younger age group in coastal Karnataka, India: A retrospective studyJ Oral Maxillofac Pathol 2018 22:180-87.10.4103/jomfp.JOMFP_16_1830158769 [Google Scholar] [CrossRef] [PubMed]
[4]. Cervantes Gracia K, Llanas-Cornejo D, Husi H, CVD and oxidative stressJ Clin Med 2017 6(2):pii: E2210.3390/jcm602002228230726 [Google Scholar] [CrossRef] [PubMed]
[5]. Panth N, Paudel KR, Parajuli K, Reactive oxygen species: A key hallmark of cardiovascular diseaseAdv Med 2016 2016:915273210.1155/2016/915273227774507 [Google Scholar] [CrossRef] [PubMed]
[6]. Kumar J, Teoh SL, Das S, Mahakknaukrauh P, Oxidative stress in oral diseases: Understanding its relation with other systemic diseasesFront Physiol 2017 8:69310.3389/fphys.2017.0069328959211 [Google Scholar] [CrossRef] [PubMed]
[7]. Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS)Redox Biol 2017 13:94-162.10.1016/j.redox.2017.05.00728577489 [Google Scholar] [CrossRef] [PubMed]
[8]. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O, Oxidative stress and antioxidant defenseWorld Allergy Organ J 2012 5(1):09-19.10.1097/WOX.0b013e318243961323268465 [Google Scholar] [CrossRef] [PubMed]
[9]. Petriello MC, Hoffman JB, Morris AJ, Hennig B, Emerging roles of xenobiotic detoxification enzymes in metabolic diseasesRev Environ Health 2017 32(1-2):105-10.10.1515/reveh-2016-005027837601 [Google Scholar] [CrossRef] [PubMed]
[10]. Mitsiogianni M, Koutsidis G, Mavroudis N, Trafalis DT, Botaitis S, Franco R, The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agentsAntioxidants (Basel) 2019 8(4):pii:E10610.3390/antiox804010631003534 [Google Scholar] [CrossRef] [PubMed]
[11]. Hodges RE, Minich DM, Modulation of metabolic detoxification pathways using foods and food-derived components: A scientific review with clinical applicationJ Nutr Metab 2015 2015:76068910.1155/2015/76068926167297 [Google Scholar] [CrossRef] [PubMed]
[12]. Ryan BM, Faupel-Badger JM, The hallmarks of premalignant conditions: A molecular basis for cancer preventionSemin Oncol 2016 43(1):22-35.10.1053/j.seminoncol.2015.09.00726970122 [Google Scholar] [CrossRef] [PubMed]
[13]. Bishayee A, Sethi G, Bioactive natural products in cancer prevention and therapy: Progress and promiseSemin Cancer Biol 2016 40-41:01-03.10.1016/j.semcancer.2016.08.00627565447 [Google Scholar] [CrossRef] [PubMed]
[14]. Seca AML, Pinto DCGA, Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic applicationInt J Mol Sci 2018 19(1)10.3390/ijms1901026329337925 [Google Scholar] [CrossRef] [PubMed]
[15]. Selvasundaram R, Manoharan S, Buddhan R, Neelakandan M, Murali Naidu R, Chemopreventive potential of esculetin in 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesisMol Cell Biochem 2018 448(1-2):145-53.10.1007/s11010-018-3321-029435870 [Google Scholar] [CrossRef] [PubMed]
[16]. Manimaran A, Manoharan S, Tumour preventive efficacy of emodin in 7,12-dimethylbenz(a)anthracene-induced oral carcinogenesis: A histopathological and biochemical approachPathol Oncol Res 2018 24(1):19-29.10.1007/s12253-017-0205-728138922 [Google Scholar] [CrossRef] [PubMed]
[17]. Baskaran N, Manoharan S, Karthikeyan S, Prabhakar MM, Chemopreventive potential of coumarin in 7, 12-dimethylbenz(a) anthracene induced hamster buccal pouch carcinogenesisAsian Pac J Cancer Prev 2012 13(10):5273-79.10.7314/APJCP.2012.13.10.527323244148 [Google Scholar] [CrossRef] [PubMed]
[18]. Alias LM, Manoharan S, Vellaichamy L, Balakrishnan S, Ramachandran CR, Protective effect of ferulic acid on 7,12-dimethylbenz(a)anthracene-induced skin carcinogenesis in Swiss albino miceExp Toxicol Pathol 2009 61(3):205-14.10.1016/j.etp.2008.09.00118845425 [Google Scholar] [CrossRef] [PubMed]
[19]. Pugalendhi P, Manoharan S, Suresh K, Baskaran N, Genistein and daidzein, in combination, protect cellular integrity during 7,12-dimethylbenz(a)anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley ratsAfr J Tradit Complement Altern Med 2011 8(2):91-97.10.4314/ajtcam.v8i2.6319622238489 [Google Scholar] [CrossRef] [PubMed]
[20]. Manoharan S, Karthikeyan S, Essa MM, Manimaran A, Selvasundram R, An overview of oral carcinogenesisInt J Nutr Pharmacol Neurol Dis 2016 6:51-62.0.4103/2231-0738.179964 [Google Scholar] [CrossRef]
[21]. Dragomanova S, Tancheva L, Georgieva M, Klisurov R, Analgesic and anti-inflammatory activity of monoterpenoid myrtenal in rodentsJ of IMAB 2019 25(1):2406-13.0.5272/jimab.2019251.2406 [Google Scholar] [CrossRef]
[22]. Lokeshkumar B, Sathishkumar V, Nandakumar N, Rengarajan T, Madankumar A, Balasubramanian MP, Anti-oxidative effect of myrtenal in prevention and treatment of colon cancer induced by 1, 2-dimethyl hydrazine (DMH) in experimental animalsBiomol Ther (Seoul) 2015 23(5):471-78.10.4062/biomolther.2015.03926336588 [Google Scholar] [CrossRef] [PubMed]
[23]. Rathinam A, Pari L, Myrtenal alleviates hyperglycaemia, hyperlipidaemia and improves pancreatic insulin level in STZ-induced diabetic ratsPharm Biol 2016 54(11):2521-27.10.3109/13880209.2016.116885227158912 [Google Scholar] [CrossRef] [PubMed]
[24]. Pari L, Rathinam A, Anti-diabetic effect of myrtenal on plasma and tissue glycoproteins components in STZ induced experimental diabetic ratsJournal of Diseases and Medicinal Plants (JDMP) 2016 2(1):11-16.10.11648/j.jdmp.s.2016020101.12 [Google Scholar] [CrossRef]
[25]. Hari Babu L, Perumal S, Balasubramanian MP, Myrtenal attenuates diethylnitrosamine-induced hepatocellular carcinoma in rats by stabilizing intrinsic antioxidants and modulating apoptotic and anti-apoptotic cascadesCell Oncol (Dordr) 2012 35(4):269-83.10.1007/s13402-012-0086-422722977 [Google Scholar] [CrossRef] [PubMed]
[26]. Babu LH, Perumal S, Balasubramanian MP, Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in ratsMol Cell Biochem 2012 369(1-2):183-93.10.1007/s11010-012-1381-022763672 [Google Scholar] [CrossRef] [PubMed]
[27]. Yagi K, Lipid peroxides and human diseasesChem Phys Lipids 1987 45(2-4):337-51.10.1016/0009-3084(87)90071-5 [Google Scholar] [CrossRef]
[28]. Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem 1979 95(2):351-55.10.1016/0003-2697(79)90738-3 [Google Scholar] [CrossRef]
[29]. Kakkar PD, Viswanathan PN, A modified spectrophotometric assay of superoxide dismutaseIndian J Biochem Biophys 1984 21(2):130-32. [Google Scholar]
[30]. Sinha AK, Colorimetric assay of catalaseAnal Biochem 1972 47(2):389-94.10.1016/0003-2697(72)90132-7 [Google Scholar] [CrossRef]
[31]. Desai ID, Vitamin E analysis methods for animal tissuesMethods Enzymol 1984 105(1):138-47.10.1016/S0076-6879(84)05019-9 [Google Scholar] [CrossRef]
[32]. Palan PR, Mikhail MS, Basu J, Romney SL, Plasma levels of antioxidant beta-carotene and alpha-tocopherol in uterine cervix dysplasias and cancerNutr Cancer 1991 15:13-20.10.1080/016355891095141062017395 [Google Scholar] [CrossRef] [PubMed]
[33]. Omaye ST, Turabull JD, Sanberlich HE, Selected methods for the determination of ascorbic acid in animal cells, tissues and fluidsMethods Enzymol 1979 62:01-11.10.1016/0076-6879(79)62181-X [Google Scholar] [CrossRef]
[34]. Beutler E, Kelly BM, The effect of sodium nitrite on red cell GSHExperientia 1963 19(2):96-97.10.1007/BF0214804213967892 [Google Scholar] [CrossRef] [PubMed]
[35]. Tietze F, Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissuesAnal Biochem 1969 27(3):502-22.10.1016/0003-2697(69)90064-5 [Google Scholar] [CrossRef]
[36]. Rotruck JT, Pope AL, Ganther HE, Selenium: Biochemical role as a component of glutathione peroxidaseScience 1973 179(4073):588-90.10.1126/science.179.4073.5884686466 [Google Scholar] [CrossRef] [PubMed]
[37]. Omura T, Sato R, The carbon monoxide binding pigment of liver microsomesJ Biol Chem 1964 239(1):2379-85. [Google Scholar]
[38]. Habig WH, Pabst MJ, Jakoby WBC, Glutathione-S-transferases: The first enzymatic step in mercapturic acid formationJ Biol Chem 1974 249(22):7130-39. [Google Scholar]
[39]. Carlberg BM, Mannervik G, Glutathione reductaseMethods Enzymol 1985 113(1):484-90.10.1016/S0076-6879(85)13062-4 [Google Scholar] [CrossRef]
[40]. Ernster L, DT-Diaphorase. In: Estabrook RW, Pullman ME (ed).Methods Enzymol 1967 10New YorkAcademic Press:309-17.10.1016/0076-6879(67)10059-1 [Google Scholar] [CrossRef]
[41]. Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Role of ROS and nutritional antioxidants in human diseasesFront Physiol 2018 9:47710.3389/fphys.2018.0047729867535 [Google Scholar] [CrossRef] [PubMed]
[42]. Vinoth A, Kowsalya R, Manoharan S, Assessment of lipid peroxidation and antioxidant status in vanillic acid treated 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesisJ Clin Diagn Res 2017 11(3):BF01-04.10.4103/0973-1482.19105730488845 [Google Scholar] [CrossRef] [PubMed]
[43]. Kesarwala AH, Krishna MC, Mitchell JB, Oxidative stress in oral diseasesOral Dis 2016 22(1):09-18.10.1111/odi.1230025417961 [Google Scholar] [CrossRef] [PubMed]
[44]. Manoharan S, Nagini S, Lipid peroxidation and antioxidant status in oral cancer patientsMed Sci Res 1994 22:291-92. [Google Scholar]
[45]. Madhulatha M, Venkateswarlu N, Das S, Vikramadity Estimations of various antioxidants in oral cancer patients in comparison with smokers and non-smokers-a biochemical studyInt J Res Med Sci 2017 5(11):4743-48.10.18203/2320-6012.ijrms20174645 [Google Scholar] [CrossRef]
[46]. Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR, Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivationBiochem J 2009 418(1):29-37.10.1042/BJ2008125818937644 [Google Scholar] [CrossRef] [PubMed]
[47]. Ayala A, Muñoz MF, Argüelles S, Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenalOxid Med Cell Longev 2014 2014:36043810.1155/2014/36043824999379 [Google Scholar] [CrossRef] [PubMed]
[48]. Reed L, Arlt VM, Phillips DH, The role of cytochrome P450 enzymes in carcinogen activation and detoxication: An in vivo-in vitro paradoxCarcinogenesis 2018 39(7):851-59.10.1093/carcin/bgy05829726902 [Google Scholar] [CrossRef] [PubMed]
[49]. Briguglio M, Hrelia S, Malaguti M, Serpe L, Canaparo R, Dell’Osso B, Food bioactive compounds and their interference in drug pharmacokinetic/pharmacodynamic profilesPharmaceutics 2018 10(4):pii:E27710.3390/pharmaceutics1004027730558213 [Google Scholar] [CrossRef] [PubMed]
[50]. Silvan S, Manoharan S, Baskaran N, Anusuya C, Karthikeyan S, Prabhakar MM, Chemopreventive potential of apigenin in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesisEuropean Journal of Pharmacology 2011 670(2-3):571-77.10.1016/j.ejphar.2011.09.17921970806 [Google Scholar] [CrossRef] [PubMed]