Introduction
Squamous Cell Carcinoma (SCC) is the most common malignancy of head and neck and approximately 90% of all the tumours arise from this anatomic structure [1,2]. In the past decade, there has been a sharp increase in the incidence of HPV positive HNSCC particularly oropharyngeal cancers [3]. The prevalence of HPV positivity in tonsil and oropharyngeal cancer is higher than that in laryngeal or oral cavity cancers [4]. The increase in incidence of HPV-related Oropharyngeal Squamous Cell Carcinoma (OPSCC) is well documented in North America as well as in Europe and Australia [5,6], but it was not as much well established in South America, Asia, and Africa [7]. The percentage of OPSCC cases that were HPV positive increased from 16.3% in the period 1984-1989 to over 70% in 2000-2004 in the United States [5,8]. WHO has published the data of lip and oral cavity cancer in India in GLOBOCON 2016. According to the published data, the incidence was decreased from 7.2% to 6.4% fron 1990 to 2016 in India [9,10]. HPV-16 is the commonest subtype in all types of HPV positive cancers and HPV-18 is the next most common subtype [4,11].
If HPV positive HNSCC is treated with only surgery or chemotherapy or radiotherapy, it has shown good prognosis [12,13]. HPV positive status detection is useful in the planning and customising patient treatment regimes. HPV+ oropharyngeal cancer patients have a number of favourable demographic features as compared to HPV negative orpharyngeal cancer patients [14], HPV-related OPSCC is extremely sensitive to radiation exposure and patients generally have good prognosis and long-term survivors [15].
Very few studies are available from India related to HPV status in head and neck cancer patients and none have been conducted in Gujarat [16]. Due to high incidence of HNSCC especially OPSCC in Gujarat, this study was carried out to find out the incidence of HPV infection, to assess the specific strain of HPV [17] and to study clinical and histopathological variables as well as to assess outcomes in patients with HPV positive cancers who were treated with a curative intent at our cancer centre.
Materials and Methods
The present study was a retrospective study of incidence and histopathological features of the HNSCC as well as to find out incidence of specific strain of HPV in reported cases. All the patients, who had undergone treatment (surgery or chemotherapy or radiotherapy) at Manibhai Shivabhai Patel Cancer Centre, Shree Krishna Hospital or any other hospital as well as traceable telephonically and were reviewed or reported at the Surgical Pathology Section of the Central Diagnostic Laboratory, Shree Krishna Hospital, Karamsad, Gujarat, India from January 2014 to January 2016. The study was ethically approved by Institutional Ethical Committee with ref no. (IEC/HMPCMCE/65/ Faculty/4/85/16). Approved study was used by medical oncologist and oncosurgeon for pre and postoperative counselling, instructions and follow-up [18].
Inclusion Criteria: First 100 cases of head and neck excisions or biopsy of patients with SCC who have been reviewed or reported, treated and traceable for follow-up during study period were selected for study.
Exclusion Criteria: Patients who did not fulfilled inclusion criteria and had undergone treatment but not traceable for follow-up were excluded from study.
Surgical Pathology
Following the receipt of surgical specimens in 10% formalin at the Surgical Pathology laboratory, patient identification and specimen verification were done. The small biopsy specimen were fixed for 4-5 hours and large biopsy were fixed for 12-14 hours in 10% neutral buffered formalin. Additional cuts were made depending on the size of the specimen. Detailed gross examination with cut surface appearance was recorded. Representative sections of tumour were submitted for paraffin block prepration and histopathological examination. The blocks were sectioned and stained with haemotoxylin and eosin stain. The histopathology requisition forms submitted along with the specimen were reviewed for intra-operative frozen section findings and the same were incorporated in the final histopathology report. Surgical pathology records pertaining to patient’s clinical details were retrieved from the laboratory information system. In case of any inadequacy in the history, the information was obtained from the patients’ medical records. In final report, details of sections submitted, histopathological findings including microscopy and macroscopy were included. If any additional findings were found, it was also mentioned.
Multiple histopathological characteristics were analysed microscopically, i.e., pattern of growth of tumour, tumour grade [19], stromal response, the degree of inflammatory response [20], lymphatic and vascular embolisation as well as for perineural invasion [21]. Histopathological staging of HNSCC patient was done according to UICC 7th AJCC staging [5]. Formalin fixed, paraffin embedded tissue was used for HPV. Representative blocks of the tumour were sent to Gene Lab, Surat for HPV DNA.
In Gene Lab, HPV detection was done by Polymerase Chain Reaction (PCR) and its genotyping by nucleotide sequencing.
Procedure
DNA isolation: DNA was extracted from paraffin block tissue by employing mini elute column and buffers supplied with the kit (Quick-DNA™ FFPE Kit, catalog no. D3067; Zymo Research Corp.) (Nanodrop Technologies Inc., Wilmington, DE). Quantity and purity of extracted DNA was checked by spectrophotometry (Nanodrop®) and DNA was stored at -20°C until PCR was performed.
Nested PCR based detection of HPV: A nested PCR was performed using MY09/11(L1 primer) as outer primers and GP5+/6+(L1 Primer) as inner primers. These L1 primer pairs amplify a 450 bp (MY09/11) and 140 bp (GP5+/6+) region of the HPV L1 gene. PCR reactions were performed according to the manufacturers’ instructions using 5 μL of extracted DNA. A brief master mix containing 2U/μL Taq polymerase, 200nM of dNTPs, buffer with 2.5mM MgCl2 and 200nM of each forward (PGMY09) and reverse (PGMY11) primers were prepared. We have used both forward as well as reverse primers for retrieving different HPV types like, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
2.1 First and second round PCR: The reaction mix for the first cycle of PCR was subjected to 35 cycles of amplification in the Thermal Cycle (Bio-Rad, Hercules, CA, USA), 1 minute denaturation at 94°C, 30 second annealing at 50°C and 30 second elongation at 72°C, with a final elongation step extended to 7 minute at 72°C for two times. To confirm absence of any such substance within the extracted DNA after second cycle, an inhibition control PCR reaction was carried out for every clinical sample with same method of nested PCR as mentioned above.
2.2 Agarose gel electrophoresis: PCR products, which was generated from second round of PCR were run on a 2% agarose gel (Promega Corporation, Madison, USA), then it was stained with ethidium bromide (0.5 μg/ml), after that it was visualised under a UV source (260 nm). It was followed by the documentation using an automated gel documentation system.
2.3 HPV genotyping: For the sample in which HPV DNA was detected, PCR products generated from nested PCR were purified, the purified PCR amplicon was then subjected to the double strand DNA sequencing using two pairs of sequencing reaction which were carried out using the Big Dye Terminator v 3.1 cycle sequencing kit (Applied Biosystems, USA) as per manufacturer’s instructions. The sequence data generated was compared with reference genotypes of HPV using multiple sequence alignment tools and genotype was assigned to the clinical samples based on the most nearest sequence similarity with the reference genotype. Positive result band on agarose gel electrophoresis means one of the HPV genotype present i.e., HPV 6, 11, 16, 18, 31, 33, 45, and 58 [Table/Fig-1].
Showed interpretation of test results of HPV DNA PCR at different bps.
| Size of the PCR amplicon (bp) | Interpretation |
|---|
| 100 | Detected: Successful extraction of DNA from the sample and absence of any PCR inhibitory molecules within the extracted DNA. |
| Not Detected: DNA extraction failed or presence of some PCR inhibitory molecules within the extracted DNA sample. Repeat the entire experiment starting from the clinical sample. |
| 144 | Detected: HPV DNA present in the clinical sample. |
| Not detected: HPV DNA present in the clinical sample but not extracted due to some inhibitory molecules. |
| 450 | Detected: Successful extraction of DNA from the sample and absence of any PCR inhibitory molecules within the extracted DNA. |
Quality of Life (QoL) Analysis
Outcome was assessed using the National Comprehensive Cancer Network/Functional Assesement of Cancer Therapy (NCCN FACT) questionnaire for head and neck cancer patients. Counselling of all 100 patients and their relatives was done, according to NCCN guideline for post-treatment care, diet and follow-up [22]. In all these patients, follow-up was done by telephonic conversation to assess outcome and for reminder of the advice given to them for care and diet during initial counselling.
Statistical Analysis
Statistical analysis was performed using a software program, STATA 14 software for Windows 7 and Microsoft Excel 2007. The correlation between HPV and HNSCC and the various histological parameters studied was analysed by univariate and multivariate logistic regression analysis.
Results
The present study was undertaken to evaluate the high risk pathological features with regards to HPV status in patients of HNSCC treated with curative intent at a rural based cancer centre in Gujarat, India.
Most of the cases of HNSCC were in the age groups between 40-60 years, but it had no relation with HPV positivity and it was not statistically significant [Table/Fig-2]. Out of 100 patients, in 11 (11%) tumour showed HPV positivity [Table/Fig-2]. To study prevalence of HPV status in HNSCC different parameters like, demographic data, behavioural status, clinical features and morphological features were studied [Table/Fig-2].
Showed frequency distribution of HPV prevalence status with clinical, histopathological, demographical and behavioural characteristics of patients by calculating p-value by STATA 14 software for Windows 7.
| No. of patients | HPV status | p-value |
|---|
| Positive | Negative |
|---|
| Total No. (11) | % | Total No. (89) | % | |
|---|
| Age (Years) |
| 20-29 | 04 | 00 | 0.00 | 04 | 4.49 | 0.123 |
| 30-39 | 26 | 03 | 27.27 | 23 | 25.84 |
| 40-49 | 26 | 03 | 27.27 | 23 | 25.84 |
| 50-59 | 21 | 02 | 18.18 | 19 | 21.34 |
| 60-69 | 16 | 01 | 9.09 | 15 | 16.85 |
| 70-79 | 05 | 02 | 18.18 | 03 | 3.37 |
| ≥80 | 02 | 00 | 0.00 | 02 | 2.24 |
| Gender |
| Male | 86 | 10 | 90.90 | 76 | 85.39 | 0.523 |
| Female | 14 | 01 | 9.09 | 13 | 14.60 |
| Tobbaco use |
| Yes | 95 | 10 | 90.90 | 85 | 95.50 | 0.449 |
| No | 05 | 01 | 9.09 | 04 | 4.49 |
| Gross apparance |
| Ulceroproliferative | 48 | 07 | 63.63 | 41 | 46.06 | 0.345 |
| Ulceroinfiltrative | 52 | 04 | 36.36 | 48 | 53.93 |
| Tumour site |
| Tongue | 27 | 03 | 27.27 | 24 | 26.96 | 0.723 |
| Buccal mucosa | 35 | 03 | 27.27 | 32 | 35.95 |
| Alveolus | 05 | 00 | 0.00 | 05 | 5.61 |
| Hard palate | 01 | 00 | 0.00 | 01 | 1.12 |
| Soft palate | 05 | 01 | 9.09 | 04 | 4.49 |
| Tonsil | 03 | 01 | 9.09 | 02 | 2.24 |
| Cheek | 01 | 00 | 0.00 | 01 | 1.12 |
| Pyriform fossa | 03 | 00 | 0.00 | 03 | 3.34 |
| Gingiva and gingivobuccal sulcus | 05 | 01 | 9.09 | 04 | 4.49 |
| Retromolar trigone | 03 | 00 | 0.00 | 03 | 3.34 |
| Maxilla | 03 | 00 | 0.00 | 03 | 3.34 |
| Floor of mouth | 01 | 00 | 0.00 | 01 | 1.12 |
| Larynx | 08 | 02 | 18.18 | 06 | 6.74 |
| Tumour size (cm) |
| ≤2 (T1) | 27 | 03 | 27.27 | 24 | 26.96 | 0.837 |
| 2-4 (T2) | 53 | 05 | 45.45 | 48 | 53.93 |
| >4 (T3) | 20 | 03 | 27.27 | 17 | 19.10 |
| Grade |
| Well differentiated | 39 | 06 | 54.54 | 33 | 37.07 | 0.414 |
| Moderate differentiated | 42 | 03 | 27.27 | 41 | 46.06 |
| Poorly differentiated | 19 | 02 | 18.18 | 17 | 19.10 |
| Nodal status |
| Yes | 34 | 04 | 36.36 | 30 | 34.48 | 0.552 |
| No | 66 | 07 | 63.63 | 59 | 52.87 |
| TNM stage |
| I | 21 | 03 | 27.27 | 19 | 21.34 | 0.776 |
| II | 18 | 02 | 18.18 | 16 | 17.97 |
| III | 13 | 02 | 18.18 | 11 | 12.35 |
| IV | 47 | 04 | 36.36 | 43 | 48.31 |
| Stromal response |
| Inflammatory | 96 | 11 | 100 | 85 | 95.50 | 0.623 |
| Desmoplastic | 04 | 00 | 0.00 | 04 | 4.49 |
| Myxoid | 00 | 00 | 0.00 | 00 | 0.00 |
| Inflammatory response |
| Mild | 32 | 05 | 45.45 | 27 | 30.33 | 0.071 |
| Moderate | 52 | 05 | 45.45 | 47 | 52.80 |
| Severe | 16 | 01 | 9.09 | 15 | 16.85 |
| Lymphovascular invasion |
| Yes | 03 | 00 | 0.00 | 03 | 3.37 | NA |
| No | 97 | 11 | 100 | 86 | 96.62 |
| Perineural invasion |
| Yes | 07 | 01 | 9.09 | 06 | 6.74 | 0.570 |
| No | 93 | 10 | 90.90 | 83 | 93.25 |
All 11 (11%) HPV positive patients were between age group of 30 years to 79 years.
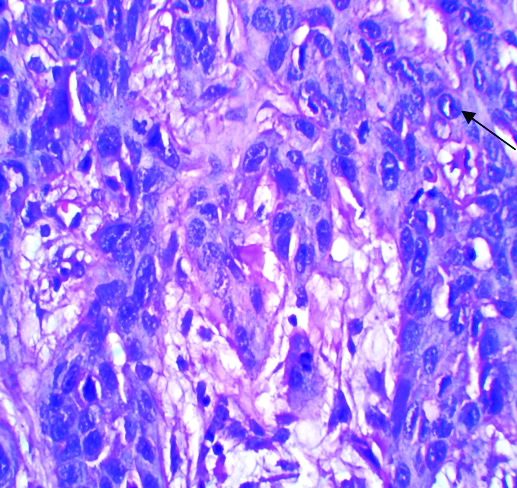
There was no age specific positivity seen in studied subject and p-value for it was 0.123, which was also statistically non significant. From 11 HPV positive patients, 10 (10%) were male and all were tobacco chewers. The p-value for both were 0.523 and 0.449, respectively. Though, it was statistically non significant, tobacco chewing was proved as one of the risk factor. Different morphological parameters were studied in all 11 HPV positive patients like; gross appearance of tumour including site and size of the tumour, microscopic grading of tumour, stromal and inflammatory response, perineural invasion and lymphnode status. For all above parameters p-value was derived and it was >0.005. This suggests that, HPV positivity has no statistical significance regarding grading of tumour but majority of HPV positive tumours were well differentiated SCC, they showed keratin pearl formation and koilocytic changes on Haematoxyllin and Eosin stained sections in microscopy [Table/Fig-3]. HPV positivity has no statistical significance with stromal response but in all positive tumour mild to moderate inflammatory stromal response was seen [Table/Fig-4,5]. Nodal metastasis and perineural invasion also have no statistical significance with HPV positivity but in all the cases no lymphnode metastasis or perineural invasion was seen. From above observation it was proved in present study that majority of HPV positive tumours were of well differentiated SCC with mild to moderate inflammatory stromal response, without perineural and lymphovascular embolisation. All were treated with surgery followed by chemotherapy. Lymphnode status was negative in all HPV positive so radiotherapy was not given to any patient. From continuous follow-up, it was also seen in present study that HPV positive patients have poor outcome, which was different from other published studies.
Koilocytic changes and keratin pearls seen in HPV positive well differentiated squamous cell carcinoma.

Intercellular bridges and mild stromal response in HPV positive moderately differentiated squamous cell carcinoma.
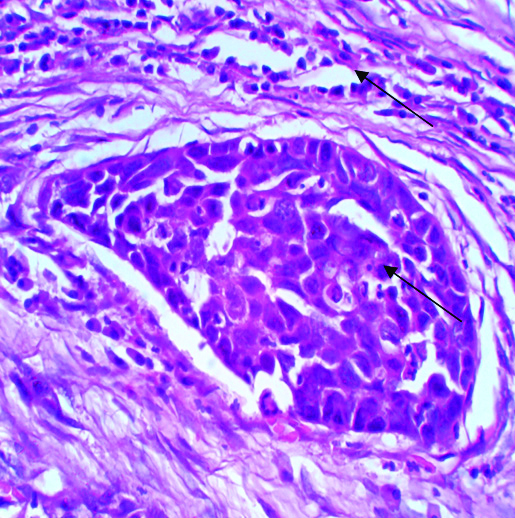
Marked nuclear pleomorphism in HPV negative poorly differentiated squamous cell carcinoma.
Discussion
Papilloma viruses are the members of the Papovaviridae family. HPV is a small, non-enveloped virus, with a diameter of 55 nm. It has an icosahedral capsid composed of 72 capsomers, which contain atleast two capsid proteins, L1 (major) and L2 (minor). Each capsomere is a pentamer of the major capsid protein and each virion capsid contains about 12 copies per virion of the minor capsid protein. The HPV genome is made up of a single molecule of double-stranded, circular DNA, which contains approximately 7.900 bp associated with histones of DNA [23].
There are more than 100 genotypes in HPV family, they are classified according to their ability to infect epithelial cells and cellular transformation [24]. Depending upon the ability of HPV to transform epithelial cells, it is divided into high risk and low risk types. Low risk types are associated with more of benign lesions like warts, while infections with high risk types progress to malignant lesions. Current test for HPV place their genotypes into low risk types (6, 11, 42, 43, 44) and high risk type (16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 59, 68) [25].
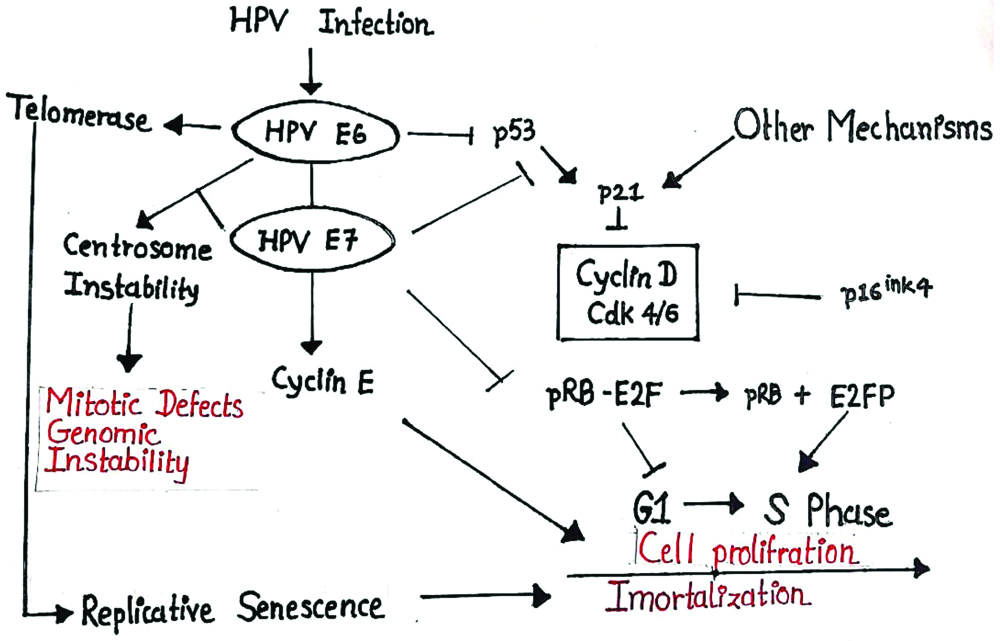
HNSCC showed heterogeneity at histological, biological and clinical level and as a result, it is difficult to predict the outcome of this malignancy. Therefore, it is crucial to find molecular markers that define tumour subgroups with homogenous behaviour and HPV may represent one of them. In normal oral mucosal epithelium, p53 expression is limited to only basal cell layer [3]. In oral epithelial dysplasia, overexpression of mutated forms of p53 is considered as high risk factor for transformation to early stage OSCC. Amongst all, different studies showed that HPV16 is the most common genome in OSCC. Inactivation of the p16 gene is frequently identified during early carcinogenesis. However, normal oral mucosa and dysplasia showed negative result immunohistochemical staining with p16 [26]. It was evident from this study that, the expression of Rb protein was found to increase from normal through the various groups, precancerous to cancerous. The overexpression seen in oral cancer, with an increase in well-differentiated and moderately differentiated tumors suggest the possible role of Rb in differentiation [Table/Fig-6] [27].
p53 HPV Rb pathway showing mechanism of HPV infection in carcinogenesis.
The prognosis and demographical characteristics of HNSCC have changed significantly over the last two decades [28-30]. The overall prevalence of HPV associated HNSCC worldwide varies from 0 to 30%. Region wise, Indian studies have reported a wide variation in prevalence of HPV associated HNSCC, ranging from 15 to 70.6% in Northeast, Eastern and Southern parts [17,31,32]. The present study found a positivity rate of 11% in the Anand district of Gujarat (Western India), which is low, as compared to other parts of India.
In the present study incidence of HPV positive HNSCC is comparable with study carried out by Portugal LG et al., (11%), Van houten VM et al., (14%), Lindel K et al., (14%), Dahlgren L et al., (9%) [33-36].
In study carried out by Brandwein-Gensler M et al., mean age was 55 years, Nasman A et al., reported mean age of 61 years, Dahlgren L et al., reported a mean age of 60 years, Attner P et al., reported mean age 62 years [20,30,36,37]. In this study, there was no age difference between patients with HPV positive and HPV negative HNSCC.
In the present study, in HPV positive HNSCC, gender difference was comparable with the study carried out by Mork J et al., Fakhry C et al., Hoffmann M et al,, and Chang JY et al., [38-41]. In above studies male and female incidence were 81% and 19%, 90% and 10%, 82% and 18%, respectively.
It was observed that tobacco consumption in any form is a risk factor and offenders have higher rate of HPV infection [Table/Fig-7] [35,39,42-45].
Showed tobacco is the major risk factor for HPV positive HNSCC by comparision with different studies [35,39,42-45].
| Sl. No. | Authors | Tobbaco |
|---|
| Yes |
|---|
| 1 | Klussman JP et al., [43] | 96% |
| 2 | Lindel K et al., [35] | 87% |
| 3 | Ritchie et al., [44] | 90% |
| 4 | Smith et al., [45] | 87% |
| 5 | Fakhry C et al., [39] | 82% |
| 6 | Liang XH et al., [42] | 87% |
| 7 | Present study | 91% |
HPV Genotype
In the present study, out of 11 HPV positive HNSCC only 1(9.09%) was HPV-16, the other were type 6, 11, 42, 43, 44 with frequency of 2(18.18%) in all. In other studies majority of HPV positive HNSCC had HPV-16 type and other high risk types, which is a discordance with the present study [13,22,46-49].
Wide variations in size of tumour were noted in the present study. The spectrum of size distribution ranged from 0.5 cm to 8.0 cm while in the other studies- majority of the tumours were in T1 and T2 category [Table/Fig-8] [30,37].
Data of comparison of size distribution in HPV positive HNSCC between different studies with present study [30,37].
| Sl. No. | Authors | Size of tumour |
|---|
| ≤2 cm (T1) | 2-4 cm (T2) | ≥4 cm (T3) | T4 |
|---|
| 1 | Nasman A et al., [30] | 25 (30%) | 40 (48%) | 13 (13%) | 5 (5%) |
| 2 | Attner P et al., [37] | 19 (27%) | 23 (32%) | 05 (7%) | 24 (34%) |
| 3 | Present study | 3 (27.27%) | 5 (45.45%) | 3 (27.27%) | 0 (0.00%) |
Some authors have combined two grades of differentiation. In the present study majority of the cases were well-differentiated, no combined grade of differentiation were used in this study [Table/Fig-9] [30,37,41,49].
Comparison of grade of differentiation in HPV positive HNSCC [30,37,41,49].
| Sl. No. | Authors | Grade of differentiation |
|---|
| Well differentiated | Moderately differentiated | Poorly differentiated |
|---|
| 1 | Chang JY et al., [41] | 29 (57%) | 16 (31%) | 6 (12%) |
| 2 | Attner P et al., [37] | 4 (6%) | 18 (25%) | 47 (66%) |
| 3 | Nassman A et al., [30] | 1 (1%) | 31 (37%) | 50 (60%) |
| 4 | Kumar B et al., [49] | 3 (100%) | 0 (0.00%) | 0 (0.00%) |
| 5 | Present study | 6 (54.54%) | 3 (27.27%) | 2 (18.18%) |
Some authors have combined two nodal stages for analysis. In the study by Chang JY et al., (82%) 42 cases were in N0 Stage and (18%) 9 in combined N1+N2+N3 Stage [41]. Fakhry C et al., quoted (34%) 13 in combined N0+N1 Stage and (66%) 25 cases in combined N2+N3 Stage [39]. Liang XH et al., (46%) 13 cases in in combined N0+N1 Stage and (54%) 15 cases in N2 Stage. Similarly few other authors had also evaluated the combined nodal status. [Table/Fig-10] [30,37,43,49].
Comparison of lymph node metastasis in HPV positive HNSCC between different studies [30,37,43,49].
| Sl. No. | Authors | Lymph node status |
|---|
| N0 | N1 | N2 | N3 |
|---|
| 1 | Klussman JP et al., [43] | 7 (30%) | 5 (21%) | 11 (49%) | 2 (9%) |
| 2 | Attner P et al., [37] | 15 (21%) | 12 (17%) | 40 (56%) | 4 (6%) |
| 3 | Kumar B et al., [49] | 0 (0.00%) | 2 (67%) | 1 (33%) | 0 (0.00%) |
| 4 | Nassman A et al., [30] | 6 (7%) | 25 (30%) | 49 (59%) | 2 (2%) |
| 5 | Present study | 7(63%) | 2(18%) | 2 (18%) | 0 (0.00%) |
Outcome
Outcome was assessed by NCCN FACT questionnaires. It suggest that the overall outcome was poor as 50% of the patients enrolled in the study had passed away by December 2015. However, for patients with HPV positive HNSCC the outcome though numerically good was statistically not significant. It is difficult to assess the association of HPV positivity and prognosis especially since majority of the patients were at a higher stage of illness as compared to those reported by other authors. Overall, prognosis in HNSCC and HPV positive HNSCC in present study was poor which was different from other published studies.
Some research groups have advocated that HPV positive and negative HNSCC cases should not only be studied as clinicopathological entities but also on the basis of response to different treatment modalities [50-52]. Several studies have shown that patients with HPV positive HNSCC have a significantly improved overall and disease free survival compared to patients with HPV negative HNSCC patients [53]. HPV positive patients have better prognosis and responded well to therapy due to fewer or different genetic mutations identified in them [52], having higher radiosensitivity, probably due to intact apoptotic response to the therapy [17,54] absence of field cancerisation [55] and immunologic response playing a role in the improved response to chemotherapy and radiotherapy [55,56].
Limitation(s)
The limitations of this study are related to the fact that all the patients of Head and Neck Squamous Cell Cancers, reported during the study period could not be included due to lack of traceability of patients or due to death of the patient happen before the study was started.
Conclusion(s)
HPV positive HNSCC reported in majority of middle aged men with habit of tobacco chewing and were of early T and N category. There was moderate aggrement found between well differentiated and keratinising SCC with HPV positivity. Though HPV related head and neck cancers have better survival rate, there was no relation found between HPV positivity with outcome of treated patients. There is limited and incomplete data available regarding the better survival rate of HPV positive head and neck cancers. In future, further studies will be needed to establish the effectiveness of HPV vaccine in prevention of head and neck cancers like it is being used in prevention of anogenital cancers.
[1]. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ, Cancer statistics, 2007CA Cancer J Clin 2017 57(1):43-66.10.3322/canjclin.57.1.4317237035 [Google Scholar] [CrossRef] [PubMed]
[2]. Vigneswaran N, Williams MD, Epidemiologic trends in head and neck cancer and aids in diagnosisOral Maxillofac Surg Clin North Am 2014 26(2):123-41.10.1016/j.coms.2014.01.00124794262 [Google Scholar] [CrossRef] [PubMed]
[3]. Kreimer AR, Clifford GM, Boyle P, Franceschi S, Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic reviewCancer Epidemiol Biomarkers Prev 2005 14(2):467-75.10.1158/1055-9965.EPI-04-055115734974 [Google Scholar] [CrossRef] [PubMed]
[4]. Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ, Human papillomavirus and head and neck cancer: A systematic review and meta-analysisClin Otolaryngol 2006 31(4):259-66.10.1111/j.1749-4486.2006.01246.x16911640 [Google Scholar] [CrossRef] [PubMed]
[5]. Chaturvedi AK, Engels EA, Pfeiffer RM, Human papillomavirus and rising oropharyngeal cancer incidence in the United StatesJ Clin Oncol 2011 29:4294-301.10.1200/JCO.2011.36.459621969503 [Google Scholar] [CrossRef] [PubMed]
[6]. Hong AM, Grulich AE, Jones D, Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targetsVaccine 2010 28:3269-72.10.1016/j.vaccine.2010.02.09820226244 [Google Scholar] [CrossRef] [PubMed]
[7]. Kristen BP, Kristina RD, Erich MS, Epidemiology of HPV-associated oropharyngeal cancerOral Oncol 2014 50(5):380-86.10.1016/j.oraloncology.2013.12.01924461628 [Google Scholar] [CrossRef] [PubMed]
[8]. Blomberg M, Nielsen A, Munk C, Kjaer SK, Trends in head and neck cancer incidence in Denmark,1978-2007: Focus on human papillomavirus associated sitesIndian J Cancer 2011 129:733-41.10.1002/ijc.2569920878955 [Google Scholar] [CrossRef] [PubMed]
[9]. Swati S, Satyanarayana L, Smitha A, Shivalingesh KK, Bala SG, Oral cancer statistics in India on the basis of first report of 29 population-based cancer registriesJ Oral Maxillofac Pathol 2018 22(1):18-26. [Google Scholar]
[10]. The Lancet Journal Available at: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(18)30447-9/fulltext; Accesed on 08.12.2016 [Google Scholar]
[11]. Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Evidence for a causal association between human papillomavirus and a subset of head and neck cancersJ Natl Cancer Inst 2000 92(9):709-20.10.1093/jnci/92.9.70910793107 [Google Scholar] [CrossRef] [PubMed]
[12]. Badaracco G, Rizzo C, Mafera B, Pichi B, Giannarelli D, Rahimi SS, Molecular analyses and prognostic relevance of HPV in head and neck tumoursOncol Rep 2007 17(4):931-40.10.3892/or.17.4.93117342339 [Google Scholar] [CrossRef] [PubMed]
[13]. Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a Non-HPV-infected cell line and relationship to signaling through AKTInt J Radiat Oncol Biol Phys 2009 74(3):928-33.10.1016/j.ijrobp.2009.03.00419480971 [Google Scholar] [CrossRef] [PubMed]
[14]. Sanders E, A comparison of clinical outcomes between HPV positive and HPV negative squamous cell carcinomas of the oropharynxORL Head Neck Nurs 2016 34(2):11-14. [Google Scholar]
[15]. De Felice F, Tombolini V, Valentini V, De Vincentiis M, Mezi S, Brugnoletti O, Advances in the management of HPV-related oropharyngeal cancerJ Clin Oncol 2019 2019:917372910.1155/2019/917372931097964 [Google Scholar] [CrossRef] [PubMed]
[16]. Karwasra RK, Sanjeev P, Sourabh N, Nisha M, Promod M, Mayank T, Human papillomavirus 16 and 18 in squamous cell carcinoma of oral cavity and sexual practices: A pilot study at a Tertiary Care Hospital of North IndiaNational. J Maxillofac Oral Surg 2015 6(2):185-89.10.4103/0975-5950.18385727390494 [Google Scholar] [CrossRef] [PubMed]
[17]. Singh AK, Kushwaha JK, Anand A, Sonkar AA, Husain N, Srivastava K, Human papilloma virus in oral cavity cancer and relation to change in quality of life following treatment-A pilot study from Northern IndiaIndian J Surg Onco 2016 7(4):386-91.10.1007/s13193-016-0559-427872524 [Google Scholar] [CrossRef] [PubMed]
[18]. Ogle OE, Postoperative care of oral and maxillofacial surgery patientsOral Maxillofac Surg Clin North Am 2006 18(1):49-58.10.1016/j.coms.2005.09.00518088810 [Google Scholar] [CrossRef] [PubMed]
[19]. Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic featuresHead Neck 2002 24(6):513-20.10.1002/hed.1009412112547 [Google Scholar] [CrossRef] [PubMed]
[20]. Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survivalAm J Surg Pathol 2005 29(2):167-78.10.1097/01.pas.0000149687.90710.2115644773 [Google Scholar] [CrossRef] [PubMed]
[21]. Jhohanese JF, Bobby C, Leon B, Frank DA, Eugene MN, Jonas TJ, Perineural invasion in squamous cell carcinoma of the head and neckArch Otolaryngol Head Neck Surg 1998 124:637-40.10.1001/archotol.124.6.6379639472 [Google Scholar] [CrossRef] [PubMed]
[22]. Gillison ML, Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entitySemin Oncol 2004 31(6):744-54.10.1053/j.seminoncol.2004.09.01115599852 [Google Scholar] [CrossRef] [PubMed]
[23]. Eileen MB, Human papillomavirus and cervical cancerClin Microbiol Rev 2003 16(1):01-17.10.1128/CMR.16.1.1-17.200312525422 [Google Scholar] [CrossRef] [PubMed]
[24]. Rajbir KK, Shamindra S, Keya S, Bhudev S, Sanjeet S, Varun R, HPV involvement in OSCC: Correlation of PCR results with light microscopic featuresJ Oral Maxillofac Pathol 2013 17(2):195-200.10.4103/0973-029X.11975624250078 [Google Scholar] [CrossRef] [PubMed]
[25]. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, A review of human carcinogens-Part B: Biological agentsLancet Oncol 2009 10(4):321-22.10.1016/S1470-2045(09)70096-8 [Google Scholar] [CrossRef]
[26]. Aline CA, Beatriz VB, Fabio DN, Eliane PD, Marcia GC, Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentially malignant disordersBraz Oral Res 2011 25(1):34-41.10.1590/S1806-8324201100010000721359449 [Google Scholar] [CrossRef] [PubMed]
[27]. Sunila T, Anita B, Prabha B, The expression of retinoblastoma tumor suppressor protein in oral cancers and precancers: A clinicopathological studyDent Res J (Isfahan) 2015 12(4):307-14.10.4103/1735-3327.16142726288619 [Google Scholar] [CrossRef] [PubMed]
[28]. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML, Incidence trends for human papillomavirus-related and-unrelated oral squamous cell carcinomas in the United StatesJ Clin Oncol 2008 26(4):612-19.10.1200/JCO.2007.14.171318235120 [Google Scholar] [CrossRef] [PubMed]
[29]. Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Joneberg J, Creson N, Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancerIndian J Cancer 2006 119(11):2620-23.10.1002/ijc.2217716991119 [Google Scholar] [CrossRef] [PubMed]
[30]. Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinomaIndian J Cancer 2009 125(2):362-66.10.1002/ijc.2433919330833 [Google Scholar] [CrossRef] [PubMed]
[31]. Mishra A, Bharti AC, Varghese P, Saluja D, Das BC, Differential expression and activation of NF-κB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infectionIndian J Cancer 2006 119(12):2840-50.10.1002/ijc.2226216998793 [Google Scholar] [CrossRef] [PubMed]
[32]. Chaudhary AK, Pandya S, Singh M, Singh M, Mehrotra R, Identification of high-risk human papillomavirus-16 and-18 infections by multiplex PCR and their expression in oral submucous fibrosis and oral squamous cell carcinomaHead Neck Oncol 2013 5(1):4 [Google Scholar]
[33]. Portugal LG, Goldenberg JD, Wenig BL, Ferrer KT, Nodzenski E, Sabnani JB, Human papillomavirus expression and p53 gene mutations in squamous cell carcinomaArch Otolaryngol Head Neck Surg 1997 123(11):1230-34.10.1001/archotol.1997.019001100840119366703 [Google Scholar] [CrossRef] [PubMed]
[34]. Van Houten VM, Snijders PJ, VandenBrekel MW, Kummer JA, Meijer CJ, Van Leeuwen B, Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomasIndian J Cancer 2001 93(2):232-35.10.1002/ijc.131311410871 [Google Scholar] [CrossRef] [PubMed]
[35]. Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM, Human papillomavirus positive squamous cell carcinoma of the oropharynxCancer 2001 92(4):805-13.10.1002/1097-0142(20010815)92:4<805::AID-CNCR1386>3.0.CO;2-9 [Google Scholar] [CrossRef]
[36]. Dahlgren L, Dahlstrand HM, Lindquist D, Högmo A, Björnestål L, Lindholm J, Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patientsIndian J Cancer 2004 112(6):1015-19.10.1002/ijc.2049015386365 [Google Scholar] [CrossRef] [PubMed]
[37]. Attner P, Du J, Näsman A, Hammarstedt L, Ramqvist T, Lindholm J, The role of human papillomavirus in the increased incidence of base of tongue cancerIndian J Cancer 2010 126(12):2879-84.10.1002/ijc.2499419856308 [Google Scholar] [CrossRef] [PubMed]
[38]. Mork J, Lie AK, Glattre E, Clark S, Hallmans G, Jellum E, Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neckN Engl J Med 2001 344(15):1125-31.10.1056/NEJM20010412344150311297703 [Google Scholar] [CrossRef] [PubMed]
[39]. Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trialJ Natl Cancer Inst 2008 100(4):261-69.10.1093/jnci/djn01118270337 [Google Scholar] [CrossRef] [PubMed]
[40]. Hoffmann M, Ihloff AS, Görögh T, Weise JB, Fazel A, Krams M, p16INK4a overexpression predicts translational active human papillomavirus infection in tonsillar cancerInian J Cancer 2010 127(7):1595-602.10.1002/ijc.2517420091864 [Google Scholar] [CrossRef] [PubMed]
[41]. Chang JY, Lin MC, Chiang CP, High-risk human papillomaviruses may have an important role in non-oral habits-associated oral squamous cell carcinomas in TaiwanAm J Clin Pathol 2003 120(6):909-16.10.1309/C5P6NUQ2NW6LCTBP14671980 [Google Scholar] [CrossRef] [PubMed]
[42]. Liang XH, Lewis J, Foote R, Smith D, Kademani D, Prevalence and significance of human papillomavirus in oral tongue cancer: The Mayo Clinic experienceJ Oral Maxillofac Surg 2008 66(9):1875-80.10.1016/j.joms.2008.04.00918718395 [Google Scholar] [CrossRef] [PubMed]
[43]. Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Eckel HE, Pfister HJ, Human papillomavirus-positive tonsillar carcinomas: A different tumor entityMed Microbiol Immunol 2003 192(3):129-32.10.1007/s00430-002-0126-112920586 [Google Scholar] [CrossRef] [PubMed]
[44]. Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynxIJC 2003 104(3):336-44.10.1002/ijc.1096012569557 [Google Scholar] [CrossRef] [PubMed]
[45]. Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancersIJC 2004 108(5):766-72.10.1002/ijc.1163314696105 [Google Scholar] [CrossRef] [PubMed]
[46]. Dahlstrand H, Näsman A, Romanitan M, Lindquist D, Ramqvist T, Dalianis T, Human papillomavirus accounts both for increased incidence and better prognosis in tonsillar cancerAnticancer Res 2008 28(2B):1133-38. [Google Scholar]
[47]. Cocks H, Ah-See K, Capel M, Taylor P, Palliative and supportive care in head and neck cancer: United Kingdom National Multidisciplinary GuidelinesJ Laryngol Otol 2016 130(S2):S198-207.10.1017/S002221511600063327841131 [Google Scholar] [CrossRef] [PubMed]
[48]. Nagel R, Martens-de Kemp SR, Buijze M, Jacobs G, Braakhuis BJ, Brakenhoff RH, Treatment response of HPV-positive and HPV-negative head and neck squamous cell carcinoma cell linesOral Oncol 2013 49(6):560-66.10.1016/j.oraloncology.2013.03.44623578372 [Google Scholar] [CrossRef] [PubMed]
[49]. Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancerJ Clin Oncol 2008 26(19):3128-37.10.1200/JCO.2007.12.766218474878 [Google Scholar] [CrossRef] [PubMed]
[50]. Sathish N, Wang X, Yuan Y, Human Papillomavirus (HPV)-associated oral cancers and treatment strategiesJ Dent Res 2014 93(7):29S-36S.10.1177/002203451452796924663683 [Google Scholar] [CrossRef] [PubMed]
[51]. Standring S, Large intestineGray’s anatomy: the anatomical basis of clinical practice 2008 40th ednPhiladelphiaChurchill Livingstone:1137 [Google Scholar]
[52]. Merati AL, Bielamowicz SA, Textbook of Laryngology 2006 Plural Publishing [Google Scholar]
[53]. Carstens MH, Neural tube programming and craniofacial cleft formation. I. The neuromeric organization of the head and neckEur J Paediatr Neurol 2004 8(4):181-210.10.1016/j.ejpn.2004.04.00315261884 [Google Scholar] [CrossRef] [PubMed]
[54]. Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH, Development of the Heart. Larsen’s Human Embryology 2015 5th edPhiladelphia, PA, USAChurchill Livingstone, an imprint of Elsevier Inc.:267-303. [Google Scholar]
[55]. Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancerArch Otolaryngol Head Neck Surg 2009 135(11):1137-46.10.1001/archoto.2009.15919917928 [Google Scholar] [CrossRef] [PubMed]
[56]. Agur AM, Dalley AF, Grant’s atlas of anatomy 2009 Lippincott Williams & Wilkins [Google Scholar]