The GIC is a commonly used restorative material in dentistry. Due to its fluoride releasing ability, it is proven as caries inhibitor [1]. However, whether this inherent property is sufficient for total arrest of secondary caries progression or not, is doubtful [2]. Therefore, therapeutic gains can be achieved by mixing antibacterial agents with GIC materials [3]. Antibiotic agents which have been proven useful in clinical dentistry, are the most appropriate choice of antibacterial agents for combining with GIC [3]. Since many years, Triple Antibiotic Paste (TAP) with metronidazole, ciprofloxacin and minocycline has been used as an intracanal medicament, against different microbes. Study done by Mittal S et al., concluded that experimental GICs containing antibiotics (ciprofloxacin, metronidazole, and minocycline) were effective in inhibiting S. mutans [4].
However, one of the major disadvantage regarding the use of TAP is tooth discolouration. Hence, application of Double Antibiotic Paste (DAP) has been considered [5,6]. Also, a number of medicinal replacements, such as Amoxicillin, Arestin, and Cefaclor have been suggested to prevent the discolouration [7]. Therefore, this study has been carried out to evaluate the antibacterial effect and microhardness of GICs containing triple antibiotic powder in different combinations, in 1.5% ratio w/w, in three experimental groups and one control group.
Materials and Methods
This is an in-vitro study, conducted during the period of three months (December 2019-February 2019) at School of dental sciences, Krishna Institute of Medical Sciences, Deemed to be University, Karad, Maharashtra, India. Study was done using set cement discs. No human tissue or teeth were used in current study. The study was approved by Institutional Ethical Research Committee (Ethical clearance protocol number: 275/2019-2020).
Sample Size
The calculated sample size was 5 samples in each group for each parameter with 90% power at 5% significance of the study. Therefore, for the four groups, a total of 40 samples were evaluated including both the parameters.
Antibacterial Cement Preparation
All antibiotics were obtained in powdered form and the group wise details are provided in [Table/Fig-1].
Antibacterial cement preparation.
| Groups | Materials | Components | Additives (w/w %) |
|---|
| Group I | Conventional restorative GIC | 10 gm of GIC (Fuji IX, GC, Tokyo, Japan) | - |
| Group II | Triple Antibiotic Powder {Metronidazole+Ciprofloxacin+Minocycline (1:1:1 ratio)} were added to GIC | 50 mg of metronidazole (J.B. Chemicals and Pharmaceuticals, India), 50 mg of ciprofloxacin (Cipla, India), 50 mg of minocycline (Sun Pharmaceuticals, India), and 9.850 g of GIC
| 1.5% |
| Group III | Triple antibiotic powder {Metronidazole+Ciprofloxacin+Cefaclor (1:1:1 ratio)} mixed with GIC | 50 mg of ciprofloxacin (Cipla, India), 50 mg of metronidazole (J.B. Chemicals and Pharmaceuticals, India), 50 mg of Cefaclor (Barqoque Pharmaceuticals, India), and 9.850 g of GIC
| 1.5% |
| Group IV | Double antibiotic powder {(Metronidazole+Ciprofloxacin (1:1 ratio)} mixed with GIC | 75 mg of ciprofloxacin (Cipla, India), 75 mg of metronidazole (J.B. Chemicals and Pharmaceuticals, India), and 9.850 g of GIC
| 1.5% |
Sample Preparation
All the powders were weighed using micro weighing machine (Wesnar, Aniso 9001:2008 Company) and mixed by the same observer. The discs measuring 10 mm in diameter and 3 mm in thickness were prepared by mixing powder and liquid from each group as per manufacturer’s instructions (P/L ratio: 3.6/1) for all the groups. Total 40 metal band moulds of desired dimensions were prepared with the help of spot welder and were used for disc preparation. Discs were allowed to set for 30 minutes before retrieving from the moulds. After the retrieval, discs were stored in de-ionised water untill further use.
Antibacterial Efficacy
The antibacterial effects against Streptococcus Mutans (a local strain obtained from the clinical samples collected in school of dental sciences, Karad, Maharashtra, India) of prepared cement discs were evaluated with agar diffusion test. The bacterial colonies were isolated from clinical samples on Tryptone Yeast Extract Cystine Sucrose Bacitracin (TYCSB) agar for specific growth. Further, suspension of the strains prepared in Phosphate Buffered Saline at 1.5×108 organisms/mL concentration by using the McFarland 0.5 turbidity tube were flood-inoculated onto the surface of freshly prepared TYCSB agar plates. Surface of the plates was air dried by leaving the specimens at 37°C for 15 minutes, after which the specimens were placed onto TYCSB agar plates [8]. After incubation at 37°C for 48-hours, inhibition zones around the specimens were measured using digital Vernier calliper in millimetres.
Microhardness Test
For microhardness testing, samples were mounted in acrylic discs. The hardness of the upper surfaces of samples were measured after polishing with aluminium oxide abrasive discs, using the Vickers microhardness measuring instrument (Mitutoyo HM-210, India). A 200-gf load was applied through the indenter with a dwell time of 10 seconds [9]. From each specimen, three readings were taken and the mean Vickers’ Hardness value was recorded.
Statistical Analysis
The post-hoc Tukey multiple-range test with one-way analysis of variance (ANOVA) was used to determine significant differences among the materials in each test at significance level of p=0.05. Statistical analyses were conducted by using INSTAT 3.1 version software.
Results
Antibacterial Efficacy
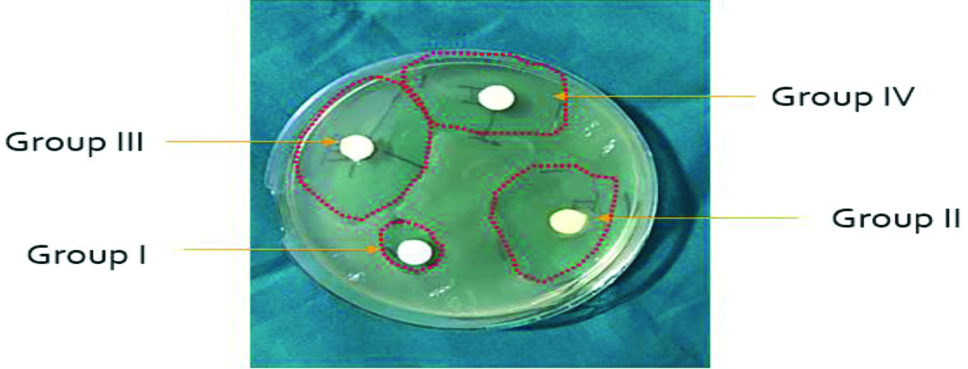
Results showing that all the groups of GICs experimented in this study showed antibacterial efficacy when tested against S. mutans [Table/Fig-2,3]. Group III (GIC containing Triple antibiotic powder with cefaclor) showed the largest diametres of the zone of growth inhibition. Group I (Control), on the other hand, presented the smallest zones of bacterial growth inhibition, probably due to fluoride release from the material. When statistically analysed using ANOVA test, the mean diameters of the zone of inhibition for S. mutans showed highly significant difference (p<0.001) amongst all three experimental groups.
Zone of inhibitions (in mm) for all the groups after 48 hours (Statistical tests employed: ANOVA test).
| Groups | Mean±SD | p-value |
|---|
| Group I (GIC) | 14.198±1.548 | p<0.001 |
| Group II (GIC+TAP containing Minocycline) | 37.312±0.878 |
| Group III (GIC+TAP containing Cefaclor) | 43.544±1.883 |
| Group IV (GIC+DAP) | 31.556±1.686 |
Diagram showing zone of inhibition of the 4 groups.
Microhardness
Mean and standard deviations of surface hardness values are presented in [Table/Fig-4]. The result of the microhardness test indicated that the microhardness value of Group III (GIC containing Triple antibiotic powder with cefaclor) was greater than that of the other GICs. Among the all GICs examined, control group showed the lowest hardness value. The differences were statistically significant (p<0.05) when the obtained microhardness values of all groups were compared using ANOVA tests.
Microhardness testing readings (in HV) (Statistical tests employed: ANOVA test)
| Groups | Mean±SD | p-value |
|---|
| Group I (GIC) | 195.68±1.567 | p<0.05 |
| Group II (GIC+TAP containing Minocycline) | 210.22±1.008 |
| Group III (GIC+TAP containing Cefaclor) | 215.24±0.978 |
| Group IV (GIC+DAP) | 189.42±0.923 |
Discussion
The effects of GIC on cariogenic bacteria are known, probably resulting from the release of fluoride, but hasn’t been proved in literature, as there are studies in contrast to this claim [10-12]. According to the results of current study, the control group also demonstrated antibacterial activity to some extent. These results are supported by previous studies claiming the antibacterial activity of GIC due to fluoride release. Study done by Hegde NN et al., [13] in 2018 concluded that the inhibitory effect of silver amalgam was the highest followed by GIC and composite, in which the growth inhibition of bacterial culture of GIC is due to the presence of fluoride.
Study done by Pinheiro SL et al., suggested the use of GIC-containing antibiotic mixture for the carious lesions treatment, which will lower the viable bacteria count [14]. Hoshino E et al., tested the antibacterial efficacy of these drugs (metronidazole, ciprofloxacin and minocycline) alone, and when used in combination, to decrease the bacteria of infected dentin [15]. Independently, none of the drugs showed complete elimination of bacteria. However, when used in combination, these drugs were able to consistently sterilise all the samples. However, one of the major concerns regarding the use of triple antibiotic powder is tooth discolouration after treatment. To eliminate this, the replacement of minocycline with cefaclor has been proved to be an alternative option [16].
Also, study done by Algarni AA et al., suggested that, use of Diantibiotic Powder (DAP) (Ciprofloxacin and Metronidazole) has shown effective antibacterial inhibitory zones against Enterococcus faecalis and Porphyromonas gingivalis [17]. Considering these previous studies, metronidazole, ciprofloxacin, minocycline and cefaclor were preferred mixture of antibiotics tested in this research. Various studies related to the current study have been summarised in [Table/Fig-5] [4,18-20]. The study done by Yesilyurt C et al., evaluated the triple antibiotic powder mixed with GIC in 1.5%, 3% and 4.5% ratio for antibacterial efficacy as well as physical strength. Results showed that, the addition of a 1.5% antibiotic mixture was capable to give appropriate physical strength as well as bonding properties [8]. Hence in the current study, the optimal concentration of 1.5% was kept constant for the combinations of triple antibiotic powder mixtures.
Various studies published in the literature [4,18-20].
| Authors and year of the study | Results |
|---|
| Ferreira JMS et al., (2013) [18] | Glass ionomer with antibiotic showed better results (82.6-95.7%) than glass ionomer (12.5-36.4%) in all evaluations (p<0.05) and the glass ionomer with antibiotics have higher difference in the success rate 46.2-72.5%. |
| Mittal S et al., (2015) [4] | All experimental groups showed inhibition against S. mutans (p<0.05), with larger zones of inhibition found in the higher concentration groups. Compressive strength at the end of 24 hours decreased in the experimental groups as compared to the control group (p<0.05), however, no difference was found between the experimental groups (p>0.05). |
| Prabhakar AR et al., (2013) [19] | Antibiotics at 1% weight solute/weight total solution after mixing may modestly confer an antibacterial activity to glass ionomer cement and enhance its fluoride-releasing ability. |
| Rahman SA et al., (2016) [20] | All experimental groups showed inhibition zone against S. mutans which was greater than that seen in control group (p<0.05). Ciprofloxacin, minocycline and combination groups showed better results compared to amoxicillin and metronidazole. |
| According to current study | All experimented groups showed inhibition against S. mutans (p<0.001) with highest diameter of zone of inhibition with group containing cefaclor |
Thibodeau B and Trope M, has used cefaclor in place of minocycline in TAP to avoid the discolouration of teeth [21]. In current study, the Group III (GIC+TAP with cefaclor) demonstrated significant reduction of biofilm formation relative to all the experimental groups tested. This proves the combination of ciprofloxacin, metronidazole and cefaclor to be more antibacterial along with eliminating the chances of discolouration. Microhardness testing is done to see material’s hardness or resistance to any deformation due to the indenting force applied to it. It is an important factor for restorative cements, to be the successful filling material in stress bearing areas without fractures. Hardness of cements can get affected with even minor changes in their powder and liquid compositions. Hence in current study, microhardness has been considered as one of the parameter along with antibacterial efficacy. This study confirms that the combinations of drugs did not affect the microhardness of the experimental materials. Moreover, it improves the material surface resistance against S. mutans. Prabhakar AR et al., added an antibiotic mixture (ciprofloxacin and metronidazole) to GIC and concluded that antibiotics at 1% enhance the antibacterial activity and fluoride release of a conventional GIC, without affecting the shear bond strength and microleakage [19].
However, even though GICs containing antibiotics are showing better caries inhibitory properties, the factor of safety should also be considered, as it may cause development of drug resistance over time. Therefore, to use the GICs modified by triple antibiotic powders, long-term clinical investigations of these material are needed. Till then, they can be used as base material, under conventional GIC materials.
Limitation(s)
The in-vitro studies have the limitation of laboratory and clinical set-up errors.
Conclusion(s)
This investigation with above limitations proved that in all the experimental groups, addition of triple antibiotic powder with cefaclor in 1.5% ratio is providing promising results with antibacterial efficacy and microhardness of the glass ionomer cement. In addition, this ratio is also not deteriorating physical strength of the cement. Further clinical studies are warranted in order to substantiate findings of this study.